Ganoderma lucidum Combined with the EGFR Tyrosine Kinase Inhibitor, Erlotinib Synergize to Reduce Inflammatory Breast Cancer Progression
- PMID: 26958085
- PMCID: PMC4780125
- DOI: 10.7150/jca.13599
Ganoderma lucidum Combined with the EGFR Tyrosine Kinase Inhibitor, Erlotinib Synergize to Reduce Inflammatory Breast Cancer Progression
Abstract
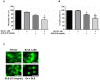
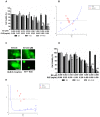
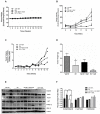
The high incidence of resistance to Tyrosine Kinase Inhibitors (TKIs) targeted against EGFR and downstream pathways has increased the necessity to identify agents that may be combined with these therapies to provide a sustained response for breast cancer patients. Here, we investigate the therapeutic potential of Ganoderma lucidum extract (GLE) in breast cancer, focusing on the regulation of the EGFR signaling cascade when treated with the EGFR TKI, Erlotinib. SUM-149, or intrinsic Erlotinib resistant MDA-MB-231 cells, and a successfully developed Erlotinib resistant cell line, rSUM-149 were treated with increasing concentrations of Erlotinib, GLE, or their combination (Erlotinib/GLE) for 72h. Treatment effects were tested on cell viability, cell proliferation, cell migration and invasion. To determine tumor progression, severe combined immunodeficient mice were injected with SUM-149 cells and then treated with Erlotinib/GLE or Erlotinib for 13 weeks. We assessed the protein expression of ERK1/2 and AKT in in vitro and in vivo models. Our results show that GLE synergizes with Erlotinib to sensitize SUM-149 cells to drug treatment, and overcomes intrinsic and developed Erlotinib resistance. Also, Erlotinib/GLE decreases SUM-149 cell viability, proliferation, migration and invasion. GLE increases Erlotinib sensitivity by inactivating AKT and ERK signaling pathways in our models. We conclude that a combinatorial therapeutic approach may be the best way to increase prognosis in breast cancer patients with EGFR overexpressing tumors.
Keywords: EGFR; Erlotinib; Ganoderma lucidum; drug resistance.; synergy.
Conflict of interest statement
Conflict of interest: The authors declare that they have no conflict of interest.
Figures


References
-
- Dawood S, Ueno NT, Valero V, Woodward WA, Buchholz TA, Hortobagyi GN. et al. Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer. 2011;117:1819–26. - PubMed
-
- Walker R. Rosen's Breast Pathology. J Clin Pathol. 1997;50:1036.
-
- Cabioglu N, Gong Y, Islam R, Broglio KR, Sneige N, Sahin A. et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2007;18:1021–9. - PubMed
-
- Yamauchi H, Ueno NT. Targeted therapy in inflammatory breast cancer. Cancer. 2010;116:2758–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

