A microfluidic device for automated, high-speed microinjection of Caenorhabditis elegans
- PMID: 26958099
- PMCID: PMC4769256
- DOI: 10.1063/1.4941984
A microfluidic device for automated, high-speed microinjection of Caenorhabditis elegans
Abstract
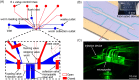
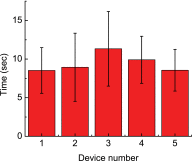
The nematode worm Caenorhabditis elegans has been widely used as a model organism in biological studies because of its short and prolific life cycle, relatively simple body structure, significant genetic overlap with human, and facile/inexpensive cultivation. Microinjection, as an established and versatile tool for delivering liquid substances into cellular/organismal objects, plays an important role in C. elegans research. However, the conventional manual procedure of C. elegans microinjection is labor-intensive and time-consuming and thus hinders large-scale C. elegans studies involving microinjection of a large number of C. elegans on a daily basis. In this paper, we report a novel microfluidic device that enables, for the first time, fully automated, high-speed microinjection of C. elegans. The device is automatically regulated by on-chip pneumatic valves and allows rapid loading, immobilization, injection, and downstream sorting of single C. elegans. For demonstration, we performed microinjection experiments on 200 C. elegans worms and demonstrated an average injection speed of 6.6 worm/min (average worm handling time: 9.45 s/worm) and a success rate of 77.5% (post-sorting success rate: 100%), both much higher than the performance of manual operation (speed: 1 worm/4 min and success rate: 30%). We conducted typical viability tests on the injected C. elegans and confirmed that the automated injection system does not impose significant adverse effect on the physiological condition of the injected C. elegans. We believe that the developed microfluidic device holds great potential to become a useful tool for facilitating high-throughput, large-scale worm biology research.
Figures





Similar articles
-
An Automated Microfluidic System for Morphological Measurement and Size-Based Sorting of C. Elegans.IEEE Trans Nanobioscience. 2019 Jul;18(3):373-380. doi: 10.1109/TNB.2019.2904009. Epub 2019 Mar 8. IEEE Trans Nanobioscience. 2019. PMID: 30869628
-
Microfluidic Device for Microinjection of Caenorhabditis elegans.Micromachines (Basel). 2020 Mar 11;11(3):295. doi: 10.3390/mi11030295. Micromachines (Basel). 2020. PMID: 32168862 Free PMC article.
-
Robotic microinjection enables large-scale transgenic studies of Caenorhabditis elegans.Nat Commun. 2024 Oct 14;15(1):8848. doi: 10.1038/s41467-024-53108-5. Nat Commun. 2024. PMID: 39397017 Free PMC article.
-
Microfluidic systems for high-throughput and high-content screening using the nematode Caenorhabditis elegans.Lab Chip. 2017 Nov 7;17(22):3736-3759. doi: 10.1039/c7lc00509a. Lab Chip. 2017. PMID: 28840220 Review.
-
Microfluidic Devices in Advanced Caenorhabditis elegans Research.Molecules. 2016 Aug 2;21(8):1006. doi: 10.3390/molecules21081006. Molecules. 2016. PMID: 27490525 Free PMC article. Review.
Cited by
-
Preface to Special Topic: Selected Papers from the 5th International Conference on Optofluidics.Biomicrofluidics. 2016 Feb 29;10(1):011701. doi: 10.1063/1.4942611. eCollection 2016 Jan. Biomicrofluidics. 2016. PMID: 27076863 Free PMC article.
-
A hybrid microfluidic device for on-demand orientation and multidirectional imaging of C. elegans organs and neurons.Biomicrofluidics. 2016 Dec 1;10(6):064111. doi: 10.1063/1.4971157. eCollection 2016 Nov. Biomicrofluidics. 2016. PMID: 27990213 Free PMC article.
-
An automated system for high-throughput generation and optimization of microdroplets.Biomicrofluidics. 2016 Sep 27;10(5):054110. doi: 10.1063/1.4963666. eCollection 2016 Sep. Biomicrofluidics. 2016. PMID: 27733891 Free PMC article.
-
Deep Learning for Microfluidic-Assisted Caenorhabditis elegans Multi-Parameter Identification Using YOLOv7.Micromachines (Basel). 2023 Jun 29;14(7):1339. doi: 10.3390/mi14071339. Micromachines (Basel). 2023. PMID: 37512650 Free PMC article.
-
Microinjection in C. elegans by direct penetration of elastomeric membranes.Biomicrofluidics. 2023 Jan 13;17(1):014103. doi: 10.1063/5.0130806. eCollection 2023 Jan. Biomicrofluidics. 2023. PMID: 36647539 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
