Role of the vagus nerve in the development and treatment of diet-induced obesity
- PMID: 26959077
- PMCID: PMC5063945
- DOI: 10.1113/JP271538
Role of the vagus nerve in the development and treatment of diet-induced obesity
Abstract
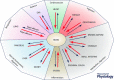
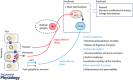
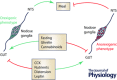
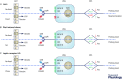
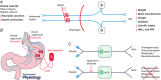
This review highlights evidence for a role of the vagus nerve in the development of obesity and how targeting the vagus nerve with neuromodulation or pharmacology can be used as a therapeutic treatment of obesity. The vagus nerve innervating the gut plays an important role in controlling metabolism. It communicates peripheral information about the volume and type of nutrients between the gut and the brain. Depending on the nutritional status, vagal afferent neurons express two different neurochemical phenotypes that can inhibit or stimulate food intake. Chronic ingestion of calorie-rich diets reduces sensitivity of vagal afferent neurons to peripheral signals and their constitutive expression of orexigenic receptors and neuropeptides. This disruption of vagal afferent signalling is sufficient to drive hyperphagia and obesity. Furthermore neuromodulation of the vagus nerve can be used in the treatment of obesity. Although the mechanisms are poorly understood, vagal nerve stimulation prevents weight gain in response to a high-fat diet. In small clinical studies, in patients with depression or epilepsy, vagal nerve stimulation has been demonstrated to promote weight loss. Vagal blockade, which inhibits the vagus nerve, results in significant weight loss. Vagal blockade is proposed to inhibit aberrant orexigenic signals arising in obesity as a putative mechanism of vagal blockade-induced weight loss. Approaches and molecular targets to develop future pharmacotherapy targeted to the vagus nerve for the treatment of obesity are proposed. In conclusion there is strong evidence that the vagus nerve is involved in the development of obesity and it is proving to be an attractive target for the treatment of obesity.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures

References
-
- Al Massadi O, Pardo M, Roca‐Rivada A, Castelao C, Casanueva FF & Seoane LM (2010). Macronutrients act directly on the stomach to regulate gastric ghrelin release. J Endocrinol Invest 33, 599–602. - PubMed
-
- Berridge KC (1996). Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev 20, 1–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

