Radiation therapy generates platelet-activating factor agonists
- PMID: 26959112
- PMCID: PMC4991492
- DOI: 10.18632/oncotarget.7878
Radiation therapy generates platelet-activating factor agonists
Abstract
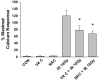
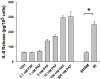
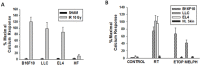
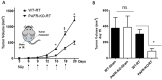
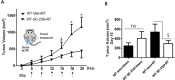
Pro-oxidative stressors can suppress host immunity due to their ability to generate oxidized lipid agonists of the platelet-activating factor-receptor (PAF-R). As radiation therapy also induces reactive oxygen species, the present studies were designed to define whether ionizing radiation could generate PAF-R agonists and if these lipids could subvert host immunity. We demonstrate that radiation exposure of multiple tumor cell lines in-vitro, tumors in-vivo, and human subjects undergoing radiation therapy for skin tumors all generate PAF-R agonists. Structural characterization of radiation-induced PAF-R agonistic activity revealed PAF and multiple oxidized glycerophosphocholines that are produced non-enzymatically. In a murine melanoma tumor model, irradiation of one tumor augmented the growth of the other (non-treated) tumor in a PAF-R-dependent process blocked by a cyclooxygenase-2 inhibitor. These results indicate a novel pathway by which PAF-R agonists produced as a byproduct of radiation therapy could result in tumor treatment failure, and offer important insights into potential therapeutic strategies that could improve the overall antitumor effectiveness of radiation therapy regimens.
Keywords: antioxidants; cyclooxygenase type 2 enzyme; oxidized glycerophosphocholines; platelet-activating factor; radiation therapy.
Conflict of interest statement
None of the authors have a relevant conflict of interest.
Figures

References
-
- Vikram B, Coleman CN, Deye JA. Current status and future potential of advanced technologies in radiation oncology Part 1. Challenges and resources. Oncology (Williston Park) 2009;23:279–83. - PubMed
-
- Tominaga H, Kodama S, Matsuda N, Suzuki K, Watanabe M. Involvement of reactive oxygen species (ROS) in the induction of genetic instability by radiation. Journal of Radiation Research. 2004;45:181–8. - PubMed
-
- Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, Inanami O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radical Biology and Medicine. 2012;53:260–70. - PubMed
-
- Ramos G, Limon-Flores AY, Ullrich SE. Dermal exposure to jet fuel suppresses delayed-type hypersensitivity: a critical role for aromatic hydrocarbons. Toxicol Sci. 2007;100:415–422. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

