Complications and Safety of Preconception Low-Dose Aspirin Among Women With Prior Pregnancy Losses
- PMID: 26959198
- PMCID: PMC4805457
- DOI: 10.1097/AOG.0000000000001301
Complications and Safety of Preconception Low-Dose Aspirin Among Women With Prior Pregnancy Losses
Abstract
Objective: To evaluate complications and safety of preconception low-dose aspirin in 1,228 U.S. women (2007-2011).
Methods: Evaluation of the safety of low-dose aspirin in the participants and their fetuses was a planned secondary analysis of the Effects of Aspirin in Gestation and Reproduction trial, a multicenter, block-randomized, double-blind, placebo-controlled trial investigating the effect of low-dose aspirin on the incidence of live birth. Women aged 18-40 years with a history of one to two pregnancy losses trying to conceive were randomized to daily low-dose aspirin (81 mg, n=615) or placebo (n=613) and were followed for up to six menstrual cycles or through gestation if they became pregnant. Emergency care visits and possible aspirin-related symptoms were assessed at each study follow-up using standardized safety interviews. In addition, complications for both the participant and her fetus or neonate were captured prospectively using case report forms, interviews conducted during pregnancy and postpartum, and medical records.
Results: The proportion of women with at least one possible aspirin-related symptom during the trial was similar between treatment arms (456 [74%] low-dose aspirin compared with 447 [73%] placebo, P=.65) as was the proportion with at least one emergency care visit (104 [17%] low-dose aspirin compared with 99 [16%] placebo, P=.76). Maternal complications were evenly distributed by treatment arm with the exception of vaginal bleeding, which was more commonly reported in the low-dose aspirin arm (22% compared with 17%, P=.02). The distribution of fetal and neonatal complications-which included three stillbirths, three neonatal deaths, and 10 neonates with birth defect(s)-was similar between treatment arms.
Conclusion: Although rare but serious complications resulting from low-dose aspirin cannot be ruled out, preconception low-dose aspirin appears to be well tolerated by women trying to conceive, women who become pregnant, and by their fetuses and neonates.
Conflict of interest statement
The authors did not report any potential conflicts of interest.
Figures
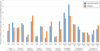
References
-
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2) Cd004659. - PubMed
-
- Henderson JT, Whitlock EP, O’Conner E, Senger CA, Thompson JH, Rowland MG. Low-Dose Aspirin for the Prevention of Morbidity and Mortality From Preeclampsia: A Systematic Evidence Review for the US Preventive Services Task Force Evidence Synthesis No 112 AHRQ Publication No 14-05207-EF-1. Rockville (MD: Agency for Healthcare Research and Quality (US); 2014. - PubMed
-
- Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013 Nov;122(5):1122–1131. - PubMed
-
- Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2014 May;45(5):1545–1588. - PMC - PubMed
-
- Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000 Oct;11(8):327–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

