Chemical and Hormonal Effects on STAT5b-Dependent Sexual Dimorphism of the Liver Transcriptome
- PMID: 26959237
- PMCID: PMC4784907
- DOI: 10.1371/journal.pone.0150284
Chemical and Hormonal Effects on STAT5b-Dependent Sexual Dimorphism of the Liver Transcriptome
Erratum in
-
Correction: Chemical and Hormonal Effects on STAT5b- Dependent Sexual Dimorphism of the Liver Transcriptome.PLoS One. 2016 Aug 16;11(8):e0161519. doi: 10.1371/journal.pone.0161519. eCollection 2016. PLoS One. 2016. PMID: 27529843 Free PMC article.
Abstract
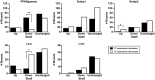
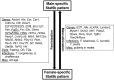
The growth hormone (GH)-activated transcription factor signal transducer and activator of transcription 5b (STAT5b) is a key regulator of sexually dimorphic gene expression in the liver. Suppression of hepatic STAT5b signaling is associated with lipid metabolic dysfunction leading to steatosis and liver cancer. In the companion publication, a STAT5b biomarker gene set was identified and used in a rank-based test to predict both increases and decreases in liver STAT5b activation status/function with high (≥ 97%) accuracy. Here, this computational approach was used to identify chemicals and hormones that activate (masculinize) or suppress (feminize) STAT5b function in a large, annotated mouse liver and primary hepatocyte gene expression compendium. Exposure to dihydrotestosterone and thyroid hormone caused liver masculinization, whereas glucocorticoids, fibroblast growth factor 15, and angiotensin II caused liver feminization. In mouse models of diabetes and obesity, liver feminization was consistently observed and was at least partially reversed by leptin or resveratrol exposure. Chemical-induced feminization of male mouse liver gene expression profiles was a relatively frequent phenomenon: of 156 gene expression biosets from chemically-treated male mice, 29% showed feminization of liver STAT5b function, while <1% showed masculinization. Most (93%) of the biosets that exhibited feminization of male liver were also associated with activation of one or more xenobiotic-responsive receptors, most commonly constitutive activated receptor (CAR) or peroxisome proliferator-activated receptor alpha (PPARα). Feminization was consistently associated with increased expression of peroxisome proliferator-activated receptor gamma (Pparg) but not other lipogenic transcription factors linked to steatosis. GH-activated STAT5b signaling in mouse liver is thus commonly altered by diverse chemicals, and provides a linkage between chemical exposure and dysregulated gene expression associated with adverse effects on the liver.
Conflict of interest statement
Figures





References
-
- Frank SJ (2001) Growth hormone signalling and its regulation: preventing too much of a good thing. Growth Horm IGF Res 11: 201–212. - PubMed
-
- Lin JX, Leonard WJ (2000) The role of STAT5a and STAT5b in signaling by IL-2 family cytokines. Oncogene 19: 2566–2576. - PubMed
-
- Herrington J, Carter-Su C (2001) Signaling pathways activated by the growth hormone receptor. Trends Endocrinol Metab 12: 252–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

