Serum Removal from Culture Induces Growth Arrest, Ploidy Alteration, Decrease in Infectivity and Differential Expression of Crucial Genes in Leishmania infantum Promastigotes
- PMID: 26959417
- PMCID: PMC4784933
- DOI: 10.1371/journal.pone.0150172
Serum Removal from Culture Induces Growth Arrest, Ploidy Alteration, Decrease in Infectivity and Differential Expression of Crucial Genes in Leishmania infantum Promastigotes
Abstract
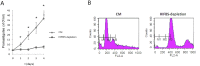
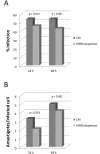
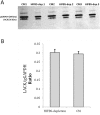
Leishmania infantum is one of the species responsible for visceral leishmaniasis. This species is distributed basically in the Mediterranean basin. A recent outbreak in humans has been reported in Spain. Axenic cultures are performed for most procedures with Leishmania spp. promastigotes. This model is stable and reproducible and mimics the conditions of the gut of the sand fly host, which is the natural environment of promastigote development. Culture media are undefined because they contain mammalian serum, which is a rich source of complex lipids and proteins. Serum deprivation slows down the growth kinetics and therefore, yield in biomass. In fact, we have confirmed that the growth rate decreases, as well as infectivity. Ploidy is also affected. Regarding the transcriptome, a high-throughput approach has revealed a low differential expression rate but important differentially regulated genes. The most remarkable profiles are: up-regulation of the GINS Psf3, the fatty acyl-CoA synthase (FAS1), the glyoxylase I (GLO1), the hydrophilic surface protein B (HASPB), the methylmalonyl-CoA epimerase (MMCE) and an amastin gene; and down-regulation of the gPEPCK and the arginase. Implications for metabolic adaptations, differentiation and infectivity are discussed herein.
Conflict of interest statement
Figures



References
-
- Arce A, Estirado A, Ordobas M, Sevilla S, Garcia N, Moratilla L, et al. Re-emergence of leishmaniasis in Spain: community outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill. 2013;18(30):20546 Epub 2013/08/10. 20546 [pii]. . - PubMed
-
- Jimenez M, Gonzalez E, Martin-Martin I, Hernandez S, Molina R. Could wild rabbits (Oryctolagus cuniculus) be reservoirs for Leishmania infantum in the focus of Madrid, Spain? Vet Parasitol. 2014;202(3–4):296–300. Epub 2014/04/30. 10.1016/j.vetpar.2014.03.027 S0304-4017(14)00202-7 [pii]. . - DOI - PubMed
-
- Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17(3):279–89. Epub 1999/06/29. S0738-081X(99)00046-2 [pii]. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

