Plasmalogen Augmentation Reverses Striatal Dopamine Loss in MPTP Mice
- PMID: 26959819
- PMCID: PMC4784967
- DOI: 10.1371/journal.pone.0151020
Plasmalogen Augmentation Reverses Striatal Dopamine Loss in MPTP Mice
Abstract
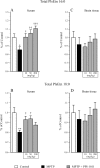
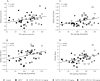
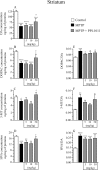
Plasmalogens are a class of glycerophospholipids shown to play critical roles in membrane structure and function. Decreased plasmalogens are reported in the brain and blood of Parkinson's disease (PD) patients. The present study investigated the hypothesis that augmenting plasmalogens could protect striatal dopamine neurons that degenerate in response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment in mice, a PD model. First, in a pre-treatment experiment male mice were treated for 10 days with the docosahexaenoic acid (DHA)-plasmalogen precursor PPI-1011 (10, 50 and 200 mg/kg). On day 5 mice received MPTP and were killed on day 11. Next, in a post-treatment study, male mice were treated with MPTP and then received daily for 5 days PPI-1011 (5, 10 and 50 mg/kg). MPTP treatment reduced serum plasmalogen levels, striatal contents of dopamine (DA) and its metabolites, serotonin, DA transporter (DAT) and vesicular monoamine transporter 2 (VMAT2). Pre-treatment with PPI-1011 (10 and 50 mg/kg) prevented all MPTP-induced effects. Positive correlations were measured between striatal DA contents and serum plasmalogen levels as well as striatal DAT and VMAT2 specific binding. Post-treatment with PPI-1011 prevented all MPTP-induced effects at 50 mg/kg but not at lower doses. Positive correlations were measured between striatal DA contents and serum plasmalogen levels as well as striatal DAT and VMAT2 specific binding in the post-treatment experiment. PPI-1011 treatment (10 days at 5, 10 and 50 mg/kg) of intact mice left unchanged striatal biogenic amine contents. These data demonstrate that treatment with a plasmalogen precursor is capable of protecting striatal dopamine markers in an animal model of PD.
Conflict of interest statement
Figures







References
-
- Bousquet M, Saint-Pierre M, Julien C, Salem N Jr, Cicchetti F, Calon F. Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson's disease. FASEB J. 2008;22(4):1213–25. fj.07-9677com [pii] 10.1096/fj.07-9677com - DOI - PubMed
-
- Han X, Holtzman DM, McKeel DW Jr. Plasmalogen deficiency in early Alzheimer's disease subjects and in animal models: molecular characterization using electrospray ionization mass spectrometry. J Neurochem. 2001;77(4):1168–80. - PubMed
-
- Farooqui AA, Farooqui T, Horrocks LA. Metabolism and Functions of Bioactive Ether Lipids in the Brain. 1st ed. ed2008 april 13 2008. 260 p.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

