Ultrasound-targeted hepatic delivery of factor IX in hemophiliac mice
- PMID: 26960037
- PMCID: PMC4891223
- DOI: 10.1038/gt.2016.23
Ultrasound-targeted hepatic delivery of factor IX in hemophiliac mice
Abstract
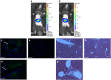
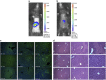
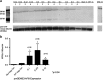
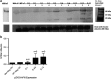
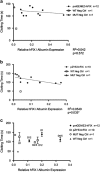
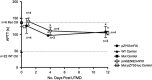
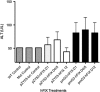
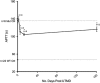
Ultrasound-targeted microbubble destruction (UTMD) was used to direct the delivery of plasmid and transposase-based vectors encoding human factor IX (hFIX) to the livers of hemophilia B (FIX-/-) mice. The DNA vectors were incorporated into cationic lipid microbubbles, injected intravenously, and transfected into hepatocytes by acoustic cavitation of the bubbles as they transited the liver. Ultrasound parameters were identified that produced transfection of hepatocytes in vivo without substantial damage or bleeding in the livers of the FIX-deficient mice. These mice were treated with a conventional expression plasmid, or one containing a piggyBac transposon construct, and hFIX levels in the plasma and liver were evaluated at multiple time points after UTMD. We detected hFIX in the plasma by western blotting from mice treated with either plasmid during the 12 days after UTMD, and in the hepatocytes of treated livers by immunofluorescence. Reductions in clotting time and improvements in the percentage of FIX activity were observed for both plasmids, conventional (4.15±1.98%), and transposon based (2.70±.75%), 4 to 5 days after UTMD compared with untreated FIX (-/-) control mice (0.92±0.78%) (P=0.001 and P=0.012, respectively). Reduced clotting times persisted for both plasmids 12 days after treatment (reflecting percentage FIX activity of 3.12±1.56%, P=0.02 and 3.08±0.10%, P=0.001, respectively). Clotting times from an additional set of mice treated with pmGENIE3-hFIX were evaluated for long-term effects and demonstrated a persistent reduction in average clotting time 160 days after a single treatment. These data suggest that UTMD could be a minimally invasive, nonviral approach to enhance hepatic FIX expression in patients with hemophilia.
Conflict of interest statement
Stefan Moisyadi is the owner of Manoa BioSciences, a start-up company out of the University of Hawaii. Manoa BioSciences holds the rights and patent for the p
Figures

Comment on
-
Adenovirus-associated virus vector-mediated gene transfer in hemophilia B.N Engl J Med. 2011 Dec 22;365(25):2357-65. doi: 10.1056/NEJMoa1108046. Epub 2011 Dec 10. N Engl J Med. 2011. PMID: 22149959 Free PMC article. Clinical Trial.
-
Long-term safety and efficacy of factor IX gene therapy in hemophilia B.N Engl J Med. 2014 Nov 20;371(21):1994-2004. doi: 10.1056/NEJMoa1407309. N Engl J Med. 2014. PMID: 25409372 Free PMC article. Clinical Trial.
References
-
- Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med 1999; 5: 64–70. - PubMed
-
- Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 1997; 16: 270–276. - PubMed
-
- Monahan PE. Factor IX: insights from knock-out and genetically engineered mice. Thromb Haemost 2008; 100: 563–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

