A Pilot Randomized Placebo Controlled Trial of Electroacupuncture for Women with Pure Stress Urinary Incontinence
- PMID: 26960195
- PMCID: PMC4784883
- DOI: 10.1371/journal.pone.0150821
A Pilot Randomized Placebo Controlled Trial of Electroacupuncture for Women with Pure Stress Urinary Incontinence
Abstract
Background: Acupuncture is a potential conservative therapy for women with stress urinary incontinence (SUI). There is limited evidence to support its effectiveness due to the poor quality of existing studies.
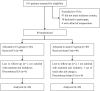
Methods: We performed a pilot randomized, controlled trial to preliminarily assess the efficacy of electroacupuncture (EA) in women with pure SUI. A total of 80 women with pure SUI were randomly assigned to receive EA with deep needling at BL33 and BL35 (n = 40) or sham EA with non-penetrating needling at sham acupoints (n = 40) three sessions per week for 6 weeks. The women were followed for 24 weeks. The primary outcome was the change from baseline in the amount of urine leakage measured by a 1-hour pad test after 6 weeks. The secondary outcomes included the 72-hour incontinence episode frequency (IEF), International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) score, and patient self-evaluation of therapeutic effect. Adverse events (AEs) were monitored throughout the trial.
Results: The median decrease from baseline of urine leakage measured by the 1-hour pad test was 2.5 g [interquartile range (IQR): 1.80-14.6 in the EA group, which was greater than the median decrease of 0.05 g (IQR: -2.80-+0.50) in the sham EA group after 6 weeks (p<0.01). The differences between groups in the decrease from baseline of 72-hour IEF became statistically significant at week 30 with a median decrease of 3.25 g (IQR: 1.25-5.69) in the EA group, and a median decrease of 1.00 g (IQR: -0.69-+2.88) in the sham EA group (p = 0.01). The participants in the EA group showed greater decreases in ICIQ-SF score and higher ratings in the help they received from the treatment than those in the sham EA group at weeks 6,18 and 30 (all p<0.05). No obvious AEs were observed in either group.
Conclusion: EA may effectively and safely relieve urinary incontinence symptoms and improve quality of life in women with pure SUI. EA demonstrated more than a placebo effect. Since this is a pilot study, results should be interpreted with caution.
Trial registration: ClinicalTrials.gov NCT02445573.
Conflict of interest statement
Figures
References
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. - PubMed
-
- Lalos O, Berglund AL, Lalos A. Impact of urinary and climacteric symptoms on social and sexual life after surgical treatment of stress urinary incontinence in women: a long-term outcome. Journal of advanced nursing. 2001;33:316–27. - PubMed
-
- Swithinbank LV, Abrams P. The impact of urinary incontinence on the quality of life of women. World journal of urology. 1999;17:225–9. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical