A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization
- PMID: 26961805
- PMCID: PMC4846562
- DOI: 10.1016/j.actbio.2016.03.002
A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization
Abstract
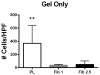
Mesenchymal stem cells (MSC) hold promise in promoting vascular regeneration of ischemic tissue in conditions like critical limb ischemia of the leg. However, this approach has been limited in part by poor cell retention and survival after delivery. New biomaterials offer an opportunity to localize cells to the desired tissue after delivery, but also to improve cell survival after delivery. Here we characterize the mechanical and microstructural properties of a novel hydrogel composed of pooled human platelet lysate (PL) and test its ability to promote MSC angiogenic activity using clinically relevant in vitro and in vivo models. This PL hydrogel had comparable storage and loss modulus and behaved as a viscoelastic solid similar to fibrin hydrogels despite having 1/4-1/10th the fibrin content of standard fibrin gels. Additionally, PL hydrogels enabled sustained release of endogenous PDGF-BB for up to 20days and were resistant to protease degradation. PL hydrogel stimulated pro-angiogenic activity by promoting human MSC growth and invasion in a 3D environment, and enhancing endothelial cell sprouting alone and in co-culture with MSCs. When delivered in vivo, the combination of PL and human MSCs improved local tissue perfusion after 8days compared to controls when assessed with laser Doppler perfusion imaging in a murine model of hind limb ischemia. These results support the use of a PL hydrogel as a scaffold for MSC delivery to promote vascular regeneration.
Statement of significance: Innovative strategies for improved retention and viability of mesenchymal stem cells (MSCs) are needed for cellular therapies. Human platelet lysate is a potent serum supplement that improves the expansion of MSCs. Here we characterize our novel PL hydrogel's desirable structural and biologic properties for human MSCs and endothelial cells. PL hydrogel can localize cells for retention in the desired tissue, improves cell viability, and augments MSCs' angiogenic activity. As a result of these unique traits, PL hydrogel is ideally suited to serve as a cell delivery vehicle for MSCs injected into ischemic tissues to promote vascular regeneration, as demonstrated here in a murine model of hindlimb ischemia.
Keywords: Angiogenesis; Cell Scaffold; Cellular therapy; Mesenchymal stem cell; Platelet lysate.
Published by Elsevier Ltd.
Conflict of interest statement
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures



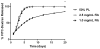
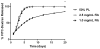
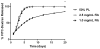
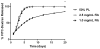
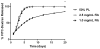
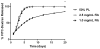
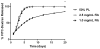
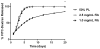
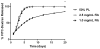
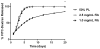




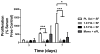
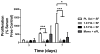







Similar articles
-
Self-assembled GFFYK peptide hydrogel enhances the therapeutic efficacy of mesenchymal stem cells in a mouse hindlimb ischemia model.Acta Biomater. 2019 Feb;85:94-105. doi: 10.1016/j.actbio.2018.12.015. Epub 2018 Dec 11. Acta Biomater. 2019. PMID: 30550934
-
Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration.Acta Biomater. 2018 Sep 1;77:155-171. doi: 10.1016/j.actbio.2018.07.035. Epub 2018 Jul 18. Acta Biomater. 2018. PMID: 30031163
-
An injectable platelet lysate-hyaluronic acid hydrogel supports cellular activities and induces chondrogenesis of encapsulated mesenchymal stem cells.Acta Biomater. 2019 Jan 1;83:233-244. doi: 10.1016/j.actbio.2018.10.031. Epub 2018 Oct 24. Acta Biomater. 2019. PMID: 30366137
-
Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future.Stem Cell Res Ther. 2016 Jul 13;7(1):93. doi: 10.1186/s13287-016-0352-x. Stem Cell Res Ther. 2016. PMID: 27411942 Free PMC article. Review.
-
Mesenchymal Stromal Cells (MSCs): A Promising Tool for Cell-Based Angiogenic Therapy.Curr Gene Ther. 2021;21(5):382-405. doi: 10.2174/1566523221666210917114353. Curr Gene Ther. 2021. PMID: 34533444 Review.
Cited by
-
Engineering Extracellular Microenvironment for Tissue Regeneration.Bioengineering (Basel). 2022 May 8;9(5):202. doi: 10.3390/bioengineering9050202. Bioengineering (Basel). 2022. PMID: 35621480 Free PMC article. Review.
-
Blood Plasma Derivatives for Tissue Engineering and Regenerative Medicine Therapies.Tissue Eng Part B Rev. 2018 Dec;24(6):454-462. doi: 10.1089/ten.TEB.2018.0008. Epub 2018 Oct 5. Tissue Eng Part B Rev. 2018. PMID: 29737237 Free PMC article. Review.
-
Hydrogel supplemented with human platelet lysate enhances multi-lineage differentiation of mesenchymal stem cells.J Nanobiotechnology. 2022 Apr 2;20(1):176. doi: 10.1186/s12951-022-01387-9. J Nanobiotechnology. 2022. PMID: 35366889 Free PMC article.
-
Hydrogel Encapsulation: Taking the Therapy of Mesenchymal Stem Cells and Their Derived Secretome to the Next Level.Front Bioeng Biotechnol. 2022 Apr 1;10:859927. doi: 10.3389/fbioe.2022.859927. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35433656 Free PMC article. Review.
-
Using biomaterials to rewire the process of wound repair.Biomater Sci. 2017 Jul 25;5(8):1421-1434. doi: 10.1039/c7bm00295e. Biomater Sci. 2017. PMID: 28692083 Free PMC article. Review.
References
-
- Fowkes FG, Housley E, Cawood EH, Macintyre CC, Ruckley CV, Prescott RJ. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. [accessed November 16, 2014];Int J Epidemiol. 1991 20:384–92. http://www.ncbi.nlm.nih.gov/pubmed/1917239. - PubMed
-
- Novo S, Avellone G, Di Garbo V, Abrignani MG, Liquori M, Panno AV, Strano A. Prevalence of risk factors in patients with peripheral arterial disease. A clinical and epidemiological evaluation. [accessed November 16, 2014];Int Angiol. 11:218–29. http://www.ncbi.nlm.nih.gov/pubmed/1460357. - PubMed
-
- Critical limb ischaemia: management and outcome. Report of a national survey. The Vascular Surgical Society of Great Britain and Ireland. [accessed November 16, 2014];Eur J Vasc Endovasc Surg. 1995 10:108–13. http://www.ncbi.nlm.nih.gov/pubmed/7633958. - PubMed
-
- Powell RJ, Simons M, Mendelsohn FO, Daniel G, Henry TD, Koga M, Morishita R, Annex BH. Results of a double-blind placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

