Feeding and Reward Are Differentially Induced by Activating GABAergic Lateral Hypothalamic Projections to VTA
- PMID: 26961951
- PMCID: PMC4783499
- DOI: 10.1523/JNEUROSCI.3799-15.2016
Feeding and Reward Are Differentially Induced by Activating GABAergic Lateral Hypothalamic Projections to VTA
Abstract
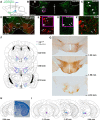
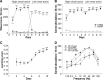
Electrical stimulation of the lateral hypothalamus (LH) has two motivational effects: long trains of stimulation induce drive-like effects such as eating, and short trains are rewarding. It has not been clear whether a single set of activated fibers subserves the two effects. Previous optogenetic stimulation studies have confirmed that reinforcement and induction of feeding can each be induced by selective stimulation of GABAergic fibers originating in the bed nucleus of the LH and projecting to the ventral tegmental area (VTA). In the present study we determined the optimal stimulation parameters for each of the two optogenetically induced effects in food-sated mice. Stimulation-induced eating was strongest with 5 Hz and progressively weaker with 10 and 20 Hz. Stimulation-induced reward was strongest with 40 Hz and progressively weaker with lower or higher frequencies. Mean preferred duration for continuous 40 Hz stimulation was 61.6 s in a "real-time" place preference task; mean preferred duration for 5 Hz stimulation was 45.6 s. The differential effects of high- and low-frequency stimulation of this pathway seem most likely to be due to differential effects on downstream targets.
Keywords: GABA; feeding; lateral hypothalamus; optogenetics; reward; ventral tegmental area.
Copyright © 2016 the authors 0270-6474/16/362975-11$15.00/0.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
