Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease
- PMID: 26962052
- PMCID: PMC5006248
- DOI: 10.1093/brain/aww027
Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease
Abstract
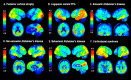
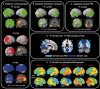
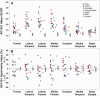
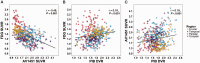
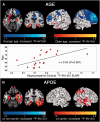
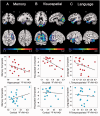
SEE SARAZIN ET AL DOI101093/BRAIN/AWW041 FOR A SCIENTIFIC COMMENTARY ON THIS ARTICLE: The advent of the positron emission tomography tracer (18)F-AV1451 provides the unique opportunity to visualize the regional distribution of tau pathology in the living human brain. In this study, we tested the hypothesis that tau pathology is closely linked to symptomatology and patterns of glucose hypometabolism in Alzheimer's disease, in contrast to the more diffuse distribution of amyloid-β pathology. We included 20 patients meeting criteria for probable Alzheimer's disease dementia or mild cognitive impairment due to Alzheimer's disease, presenting with a variety of clinical phenotypes, and 15 amyloid-β-negative cognitively normal individuals, who underwent (18)F-AV1451 (tau), (11)C-PiB (amyloid-β) and (18)F-FDG (glucose metabolism) positron emission tomography, apolipoprotein E (APOE) genotyping and neuropsychological testing. Voxel-wise contrasts against controls (at P < 0.05 family-wise error corrected) showed that (18)F-AV1451 and (18)F-FDG patterns in patients with posterior cortical atrophy ('visual variant of Alzheimer's disease', n = 7) specifically targeted the clinically affected posterior brain regions, while (11)C-PiB bound diffusely throughout the neocortex. Patients with an amnestic-predominant presentation (n = 5) showed highest (18)F-AV1451 retention in medial temporal and lateral temporoparietal regions. Patients with logopenic variant primary progressive aphasia ('language variant of Alzheimer's disease', n = 5) demonstrated asymmetric left greater than right hemisphere (18)F-AV1451 uptake in three of five patients. Across 30 FreeSurfer-defined regions of interest in 16 Alzheimer's disease patients with all three positron emission tomography scans available, there was a strong negative association between (18)F-AV1451 and (18)F-FDG uptake (Pearson's r = -0.49 ± 0.07, P < 0.001) and less pronounced positive associations between (11)C-PiB and (18)F-FDG (Pearson's r = 0.16 ± 0.09, P < 0.001) and (18)F-AV1451 and (11)C-PiB (Pearson's r = 0.18 ± 0.09, P < 0.001). Voxel-wise linear regressions thresholded at P < 0.05 (uncorrected) showed that, across all patients, younger age was associated with greater (18)F-AV1451 uptake in wide regions of the neocortex, while older age was associated with increased (18)F-AV1451 in the medial temporal lobe. APOE ϵ4 carriers showed greater temporal and parietal (18)F-AV1451 uptake than non-carriers. Finally, worse performance on domain-specific neuropsychological tests was associated with greater (18)F-AV1451 uptake in key regions implicated in memory (medial temporal lobes), visuospatial function (occipital, right temporoparietal cortex) and language (left > right temporoparietal cortex). In conclusion, tau imaging-contrary to amyloid-β imaging-shows a strong regional association with clinical and anatomical heterogeneity in Alzheimer's disease. Although preliminary, these results are consistent with and expand upon findings from post-mortem, animal and cerebrospinal fluid studies, and suggest that the pathological aggregation of tau is closely linked to patterns of neurodegeneration and clinical manifestations of Alzheimer's disease.
Keywords: APOE; AV1451 PET; Alzheimer’s disease; cognition; tau.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Distinct tau PET imaging patterns in typical and atypical Alzheimer's disease.Brain. 2016 May;139(Pt 5):1321-4. doi: 10.1093/brain/aww041. Brain. 2016. PMID: 27189580 No abstract available.
References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 270–9. - PMC - PubMed
-
- Armstrong MJ. Diagnosis and treatment of corticobasal degeneration. Curr Treat Options Neurol 2014; 16: 282. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 1992; 42: 631–9. - PubMed
-
- Barthel H, Gertz HJ, Dresel S, Peters O, Bartenstein P, Buerger K, et al. Cerebral amyloid-beta PET with florbetaben (18F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 2011; 10: 424–35. - PubMed
-
- Beffert U, Poirier J. Apolipoprotein E, plaques, tangles and cholinergic dysfunction in Alzheimer's disease. Ann N Y Acad Sci 1996; 777: 166–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

