Vaccination with Live Attenuated Simian Immunodeficiency Virus (SIV) Protects from Mucosal, but Not Necessarily Intravenous, Challenge with a Minimally Heterologous SIV
- PMID: 26962218
- PMCID: PMC4886799
- DOI: 10.1128/JVI.00192-16
Vaccination with Live Attenuated Simian Immunodeficiency Virus (SIV) Protects from Mucosal, but Not Necessarily Intravenous, Challenge with a Minimally Heterologous SIV
Abstract
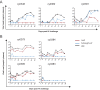
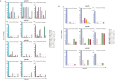
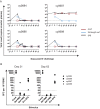
Few studies have evaluated the impact of the viral challenge route on protection against a heterologous simian immunodeficiency virus (SIV) challenge. We vaccinated seven macaques with a live attenuated SIV that differed from SIVmac239Δnef by 24 amino acids, called m3KOΔnef. All animals were protected from an intrarectal SIVmac239 challenge, whereas only four animals were protected from subsequent intravenous SIVmac239 challenge. These data suggest that immune responses elicited by vaccination with live attenuated SIV in an individual animal can confer protection from intrarectal challenge while remaining insufficient for protection from intravenous challenge.
Importance: Our study is important because we show that vaccinated animals can be protected from a mucosal challenge with a heterologous SIV, but the same animals are not necessarily protected from intravenous challenge with the same virus. This is unique because in most studies, either vaccinated animals are challenged multiple times by the same route or only a single challenge is performed. An individually vaccinated animal is rarely challenged multiple times by different routes, so protection from different challenge routes cannot be measured in the same animal. Our data imply that vaccine-elicited responses in an individual animal may be insufficient for protection from intravenous challenge but may be suitable for protection from a mucosal challenge that better approximates human immunodeficiency virus (HIV) exposure.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Berry N, Ham C, Mee ET, Rose NJ, Mattiuzzo G, Jenkins A, Page M, Elsley W, Robinson M, Smith D, Ferguson D, Towers G, Almond N, Stebbings R. 2011. Early potent protection against heterologous SIVsmE660 challenge following live attenuated SIV vaccination in Mauritian cynomolgus macaques. PLoS One 6:e23092. doi: 10.1371/journal.pone.0023092. - DOI - PMC - PubMed
-
- Reynolds MR, Weiler AM, Weisgrau KL, Piaskowski SM, Furlott JR, Weinfurter JT, Kaizu M, Soma T, Leon EJ, MacNair C, Leaman DP, Zwick MB, Gostick E, Musani SK, Price DA, Friedrich TC, Rakasz EG, Wilson NA, McDermott AB, Boyle R, Allison DB, Burton DR, Koff WC, Watkins DI. 2008. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J Exp Med 205:2537–2550. doi: 10.1084/jem.20081524. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

