Epilepsy, Behavioral Abnormalities, and Physiological Comorbidities in Syntaxin-Binding Protein 1 (STXBP1) Mutant Zebrafish
- PMID: 26963117
- PMCID: PMC4786103
- DOI: 10.1371/journal.pone.0151148
Epilepsy, Behavioral Abnormalities, and Physiological Comorbidities in Syntaxin-Binding Protein 1 (STXBP1) Mutant Zebrafish
Abstract
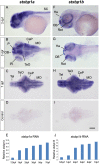
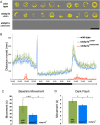
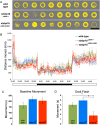
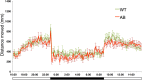
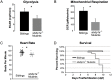
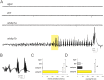
Mutations in the synaptic machinery gene syntaxin-binding protein 1, STXBP1 (also known as MUNC18-1), are linked to childhood epilepsies and other neurodevelopmental disorders. Zebrafish STXBP1 homologs (stxbp1a and stxbp1b) have highly conserved sequence and are prominently expressed in the larval zebrafish brain. To understand the functions of stxbp1a and stxbp1b, we generated loss-of-function mutations using CRISPR/Cas9 gene editing and studied brain electrical activity, behavior, development, heart physiology, metabolism, and survival in larval zebrafish. Homozygous stxbp1a mutants exhibited a profound lack of movement, low electrical brain activity, low heart rate, decreased glucose and mitochondrial metabolism, and early fatality compared to controls. On the other hand, homozygous stxbp1b mutants had spontaneous electrographic seizures, and reduced locomotor activity response to a movement-inducing "dark-flash" visual stimulus, despite showing normal metabolism, heart rate, survival, and baseline locomotor activity. Our findings in these newly generated mutant lines of zebrafish suggest that zebrafish recapitulate clinical phenotypes associated with human syntaxin-binding protein 1 mutations.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

