Development of purification processes for fully human bispecific antibodies based upon modification of protein A binding avidity
- PMID: 26963837
- PMCID: PMC4966828
- DOI: 10.1080/19420862.2016.1160192
Development of purification processes for fully human bispecific antibodies based upon modification of protein A binding avidity
Abstract
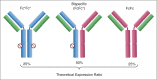
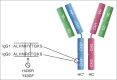
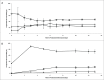
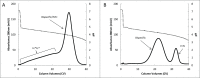
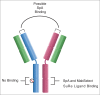
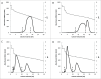
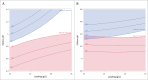
There is strong interest in the design of bispecific monoclonal antibodies (bsAbs) that can simultaneously bind 2 distinct targets or epitopes to achieve novel mechanisms of action and efficacy. Multiple bispecific formats have been proposed and are currently under development. Regeneron's bispecific technology is based upon a standard fully human IgG antibody in order to minimize immunogenicity and improve the pharmacokinetic profile. A single common light chain and 2 distinct heavy chains combine to form the bispecific molecule. One of the heavy chains contains a chimeric Fc sequence form (called Fc*) that ablates binding to Protein A via the constant region. As a result of co-expression of the 2 heavy chains and the common light chain, 3 products are created, 2 of which are homodimeric for the heavy chains and one that is the desired heterodimeric bispecific product. The Fc* sequence allows selective purification of the FcFc* bispecific product on commercially available affinity columns, due to intermediate binding affinity for Protein A compared to the high avidity FcFc heavy chain homodimer, or the weakly binding Fc*Fc* homodimer. This platform requires the use of Protein A chromatography in both a capture and polishing modality. Several challenges, including variable region Protein A binding, resin selection, selective elution optimization, and impacts upon subsequent non-affinity downstream unit operations, were addressed to create a robust and selective manufacturing process.
Keywords: Affinity chromatography; bispecific antibody; downstream process; elution pH; heterodimeric antibody; protein A chromatography; protein purification.
Figures


References
-
- Byrne H, Conroy PJ, Whisstock JC, O'Kennedy RJ. A tale of two specificities: bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol 2013; 31:621-32; PMID:24094861; http://dx.doi.org/ 10.1016/j.tibtech.2013.08.007 - DOI - PMC - PubMed
-
- May C, Sapra P, Gerber HP. Advances in bispecific biotherapeutics for the treatment of cancer. Biochem Pharmacol 2012; 84:1105-12; PMID:22858161; http://dx.doi.org/ 10.1016/j.bcp.2012.07.011 - DOI - PubMed
-
- Kontermann R. Dual targeting strategies with bispecific antibodies. mAbs 2012; 4; PMID:22453100; http://dx.doi.org/ 10.4161/mabs.4.2.19000 - DOI - PMC - PubMed
-
- Demarest SJ, Hariharan K, Dong J. Emerging antibody combinations in oncology. mAbs 2011; 3:338-51; PMID:21697653; http://dx.doi.org/ 10.4161/mabs.3.4.16615 - DOI - PMC - PubMed
-
- Muller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: current perspectives. BioDrugs: Clin Immunotherap, Biopharmac Gene Ther 2010; 24:89-98; PMID:20199124; http://dx.doi.org/ 10.2165/11530960-000000000-00000 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
