Safety of Gadobutrol: Results From 42 Clinical Phase II to IV Studies and Postmarketing Surveillance After 29 Million Applications
- PMID: 26964075
- PMCID: PMC4982758
- DOI: 10.1097/RLI.0000000000000270
Safety of Gadobutrol: Results From 42 Clinical Phase II to IV Studies and Postmarketing Surveillance After 29 Million Applications
Abstract
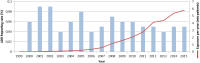
Objective: The aim of this study was to provide a systematic safety analysis of gadobutrol after more than 29 million applications in clinical routine.
Materials and methods: Forty-two clinical development phase II to IV studies on gadobutrol or comparator and the postmarketing safety surveillance database for gadobutrol (1998-2015) were analyzed. Adverse events (AEs) and drug-related AEs were evaluated in the clinical development database and spontaneous adverse drug reactions (ADRs) in the postmarketing database. Subgroup analyses were run on patients with special medical history and on patients of different age groups.
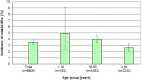
Results: In the clinical development studies, 6809 and 2184 patients received gadobutrol or comparators, respectively. The incidence of drug-related AEs was 3.5% for both groups. With the exception of nausea (0.7% related cases in both groups), all other drug-related AEs were 0.3% or less in both groups. Hypersensitivity reactions were sporadic (<0.1%). Patients with history of allergies to contrast agents experienced slightly more drug-related AEs. No differences were seen between age groups.The overall reporting rate of ADRs from postmarketing surveillance was 0.05%. The most frequent ADRs were anaphylactoid/hypersensitivity reactions, nausea, vomiting, and dyspnea.For 3 single-agent reports of nephrogenic systemic fibrosis, using a conservative approach, association with gadobutrol could not be excluded.
Conclusions: Gadobutrol is well tolerated and has a favorable safety profile for patients of all age groups.
Conflict of interest statement
Conflict of interest and sources of funding: All authors are employees of Bayer Pharma AG.
Figures
References
-
- EMC. The electronic Medicines Compendium (eMC). Available at: http://www.medicines.org.uk/emc/medicine/27209. Accessed December 12, 2015.
-
- Scott LJ. Gadobutrol: a review of its use for contrast-enhanced magnetic resonance imaging in adults and children. Clin Drug Investig. 2013;33:303–314. - PubMed
-
- Saake M, Langner S, Schwenke C, et al. MRI in multiple sclerosis: an intra-individual, randomized and multicentric comparison of gadobutrol with gadoterate meglumine at 3 T. Eur Radiol. 2016;26:820–828. - PubMed
-
- Shen Y, Goerner FL, Snyder C, et al. T1 relaxivities of gadolinium-based magnetic resonance contrast agents in human whole blood at 1.5, 3, and 7 T. Invest Radiol. 2015;50:330–338. - PubMed
-
- Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources