YiXin-Shu, a ShengMai-San-based traditional Chinese medicine formula, attenuates myocardial ischemia/reperfusion injury by suppressing mitochondrial mediated apoptosis and upregulating liver-X-receptor α
- PMID: 26964694
- PMCID: PMC4786861
- DOI: 10.1038/srep23025
YiXin-Shu, a ShengMai-San-based traditional Chinese medicine formula, attenuates myocardial ischemia/reperfusion injury by suppressing mitochondrial mediated apoptosis and upregulating liver-X-receptor α
Abstract
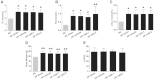
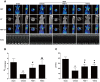
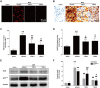
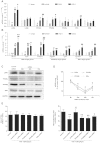
Positive evidence from clinical trials has fueled growing acceptance of traditional Chinese medicine (TCM) for the treatment of cardiac diseases; however, little is known about the underlying mechanisms. Here, we investigated the nature and underlying mechanisms of the effects of YiXin-Shu (YXS), an antioxidant-enriched TCM formula, on myocardial ischemia/reperfusion (MI/R) injury. YXS pretreatment significantly reduced infarct size and improved viable myocardium metabolism and cardiac function in hypercholesterolemic mice. Mechanistically, YXS attenuated myocardial apoptosis by inhibiting the mitochondrial mediated apoptosis pathway (as reflected by inhibition of mitochondrial swelling, cytochrome c release and caspase-9 activity, and normalization of Bcl-2 and Bax levels) without altering the death receptor and endoplasmic reticulum-stress death pathways. Moreover, YXS reduced oxidative/nitrative stress (as reflected by decreased superoxide and nitrotyrosine content and normalized pro- and anti-oxidant enzyme levels). Interestingly, YXS upregulated endogenous nuclear receptors including LXRα, PPARα, PPARβ and ERα, and in-vivo knockdown of cardiac-specific LXRα significantly blunted the cardio-protective effects of YXS. Collectively, these data show that YXS is effective in mitigating MI/R injury by suppressing mitochondrial mediated apoptosis and oxidative stress and by upregulating LXRα, thereby providing a rationale for future clinical trials and clinical applications.
Figures



Similar articles
-
Cardioprotection by combination of three compounds from ShengMai preparations in mice with myocardial ischemia/reperfusion injury through AMPK activation-mediated mitochondrial fission.Sci Rep. 2016 Nov 21;6:37114. doi: 10.1038/srep37114. Sci Rep. 2016. PMID: 27869201 Free PMC article.
-
Yixin-Shu Capsules Ameliorated Ischemia-Induced Heart Failure by Restoring Trx2 and Inhibiting JNK/p38 Activation.Oxid Med Cell Longev. 2021 Feb 16;2021:8049079. doi: 10.1155/2021/8049079. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33643519 Free PMC article.
-
Globular adiponectin attenuates myocardial ischemia/reperfusion injury by upregulating endoplasmic reticulum Ca²⁺-ATPase activity and inhibiting endoplasmic reticulum stress.J Cardiovasc Pharmacol. 2013 Aug;62(2):143-53. doi: 10.1097/FJC.0b013e31829521af. J Cardiovasc Pharmacol. 2013. PMID: 23609327
-
Research Progress of Chinese Medicine in the Treatment of Myocardial Ischemia-Reperfusion Injury.Am J Chin Med. 2023;51(1):1-17. doi: 10.1142/S0192415X23500015. Epub 2022 Nov 28. Am J Chin Med. 2023. PMID: 36437553 Review.
-
Brain oxidative stress as basic target of antioxidant traditional oriental medicines.Neurochem Res. 2009 Apr;34(4):711-6. doi: 10.1007/s11064-008-9872-9. Epub 2008 Nov 6. Neurochem Res. 2009. PMID: 18987970 Review.
Cited by
-
Yixinshu capsule combined with conventional treatment for chronic heart failure: Protocol for a systematic review and trial sequential analysis.Medicine (Baltimore). 2019 May;98(19):e14960. doi: 10.1097/MD.0000000000014960. Medicine (Baltimore). 2019. PMID: 31083149 Free PMC article.
-
Screening and identification of critical transcription factors involved in the protection of cardiomyocytes against hydrogen peroxide-induced damage by Yixin-shu.Sci Rep. 2017 Oct 24;7(1):13867. doi: 10.1038/s41598-017-10131-5. Sci Rep. 2017. PMID: 29066842 Free PMC article.
-
Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats.J Nanobiotechnology. 2019 Jan 25;17(1):18. doi: 10.1186/s12951-019-0451-9. J Nanobiotechnology. 2019. PMID: 30683110 Free PMC article.
-
Cardioprotection by combination of three compounds from ShengMai preparations in mice with myocardial ischemia/reperfusion injury through AMPK activation-mediated mitochondrial fission.Sci Rep. 2016 Nov 21;6:37114. doi: 10.1038/srep37114. Sci Rep. 2016. PMID: 27869201 Free PMC article.
-
Correlation between Mitochondrial Dysfunction, Cardiovascular Diseases, and Traditional Chinese Medicine.Evid Based Complement Alternat Med. 2020 Oct 7;2020:2902136. doi: 10.1155/2020/2902136. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33101442 Free PMC article.
References
-
- Pu J. et al. Insights into echo-attenuated plaques, echolucent plaques, and plaques with spotty calcification: novel findings from comparisons among intravascular ultrasound, near-infrared spectroscopy, and pathological histology in 2,294 human coronary artery segments. J Am Coll Cardiol. 63, 2220–2233 (2014). - PubMed
-
- Nduhirabandi F., Huisamen B., Strijdom H., Blackhurst D. & Lochner A. Short-term melatonin consumption protects the heart of obese rats independent of body weight change and visceral adiposity. J Pineal Res. 57, 317–332 (2014). - PubMed
-
- Yang Y. et al. A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J Pineal Res. 57, 357–366 (2014). - PubMed
-
- Yu L. et al. Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res. 59, 376–390 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

