Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism
- PMID: 26964832
- PMCID: PMC4892882
- DOI: 10.1038/emm.2016.4
Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism
Abstract
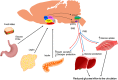
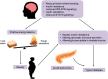
Accumulated evidence from genetic animal models suggests that the brain, particularly the hypothalamus, has a key role in the homeostatic regulation of energy and glucose metabolism. The brain integrates multiple metabolic inputs from the periphery through nutrients, gut-derived satiety signals and adiposity-related hormones. The brain modulates various aspects of metabolism, such as food intake, energy expenditure, insulin secretion, hepatic glucose production and glucose/fatty acid metabolism in adipose tissue and skeletal muscle. Highly coordinated interactions between the brain and peripheral metabolic organs are critical for the maintenance of energy and glucose homeostasis. Defective crosstalk between the brain and peripheral organs contributes to the development of obesity and type 2 diabetes. Here we comprehensively review the above topics, discussing the main findings related to the role of the brain in the homeostatic regulation of energy and glucose metabolism.
Figures

References
-
- Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol 2008; 70: 513–535. - PubMed
-
- Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science 2005; 307: 375–379. - PubMed
-
- Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol 1976; 166: 257–283. - PubMed
-
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000; 404: 661–671. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

