αv Integrins combine with LC3 and atg5 to regulate Toll-like receptor signalling in B cells
- PMID: 26965188
- PMCID: PMC4792966
- DOI: 10.1038/ncomms10917
αv Integrins combine with LC3 and atg5 to regulate Toll-like receptor signalling in B cells
Abstract
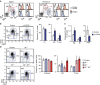
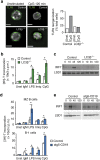
Integrin signalling triggers cytoskeletal rearrangements, including endocytosis and exocytosis of integrins and other membrane proteins. In addition to recycling integrins, this trafficking can also regulate intracellular signalling pathways. Here we describe a role for αv integrins in regulating Toll-like receptor (TLR) signalling by modulating intracellular trafficking. We show that deletion of αv or β3 causes increased B-cell responses to TLR stimulation in vitro, and αv-conditional knockout mice have elevated antibody responses to TLR-ligand-associated antigens. αv regulates TLR signalling by promoting recruitment of the autophagy component LC3 (microtubule-associated proteins 1 light chain 3) to TLR-containing endosomes, which is essential for progression from NF-κB to IRF signalling, and ultimately for traffic to lysosomes where signalling is terminated. Disruption of LC3 recruitment leads to prolonged NF-κB signalling and increased B-cell proliferation and antibody production. This work identifies a previously unrecognized role for αv and the autophagy components LC3 and atg5 in regulating TLR signalling and B-cell immunity.
Figures






Similar articles
-
αv Integrins regulate germinal center B cell responses through noncanonical autophagy.J Clin Invest. 2018 Aug 31;128(9):4163-4178. doi: 10.1172/JCI99597. Epub 2018 Aug 20. J Clin Invest. 2018. PMID: 29999501 Free PMC article.
-
Integrin-nucleated Toll-like receptor (TLR) dimerization reveals subcellular targeting of TLRs and distinct mechanisms of TLR4 activation and signaling.FEBS Lett. 2002 Dec 4;532(1-2):171-6. doi: 10.1016/s0014-5793(02)03669-4. FEBS Lett. 2002. PMID: 12459484
-
B cell autophagy mediates TLR7-dependent autoimmunity and inflammation.Autophagy. 2015;11(7):1010-24. doi: 10.1080/15548627.2015.1052206. Autophagy. 2015. PMID: 26120731 Free PMC article.
-
Toll-like receptor signaling in the lysosomal pathways.Immunol Rev. 2009 Jan;227(1):203-20. doi: 10.1111/j.1600-065X.2008.00732.x. Immunol Rev. 2009. PMID: 19120486 Review.
-
Integration of B cell responses through Toll-like receptors and antigen receptors.Nat Rev Immunol. 2012 Mar 16;12(4):282-94. doi: 10.1038/nri3190. Nat Rev Immunol. 2012. PMID: 22421786 Free PMC article. Review.
Cited by
-
Human ITGAV variants are associated with immune dysregulation, brain abnormalities, and colitis.J Exp Med. 2024 Dec 2;221(12):e20240546. doi: 10.1084/jem.20240546. Epub 2024 Nov 11. J Exp Med. 2024. PMID: 39526957 Free PMC article.
-
Genome-Wide ChIP-seq and RNA-seq Analyses of STAT3 Target Genes in TLRs Activated Human Peripheral Blood B Cells.Front Immunol. 2022 Mar 8;13:821457. doi: 10.3389/fimmu.2022.821457. eCollection 2022. Front Immunol. 2022. PMID: 35345674 Free PMC article.
-
The metabolic regulator Lamtor5 suppresses inflammatory signaling via regulating mTOR-mediated TLR4 degradation.Cell Mol Immunol. 2020 Oct;17(10):1063-1076. doi: 10.1038/s41423-019-0281-6. Epub 2019 Aug 29. Cell Mol Immunol. 2020. PMID: 31467416 Free PMC article.
-
Macropinocytosis and autophagy crosstalk in nutrient scavenging.Philos Trans R Soc Lond B Biol Sci. 2019 Feb 4;374(1765):20180154. doi: 10.1098/rstb.2018.0154. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30967004 Free PMC article.
-
Fas ligand promotes an inducible TLR-dependent model of cutaneous lupus-like inflammation.J Clin Invest. 2018 Jul 2;128(7):2966-2978. doi: 10.1172/JCI98219. Epub 2018 Jun 11. J Clin Invest. 2018. PMID: 29889098 Free PMC article.
References
-
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002) . - PubMed
-
- Bader B. L., Rayburn H., Crowley D. & Hynes R. O. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95, 507–519 (1998) . - PubMed
-
- Lacy-Hulbert A. et al.. Beta 3 integrins regulate lymphocyte migration and cytokine responses in heart transplant rejection. Am. J. Transplant. 7, 1080–1090 (2007) . - PubMed
-
- Savill J., Dransfield I., Hogg N. & Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 343, 170–173 (1990) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

