MIM regulates the trafficking of bone marrow cells via modulating surface expression of CXCR4
- PMID: 26965284
- PMCID: PMC4889520
- DOI: 10.1038/leu.2016.39
MIM regulates the trafficking of bone marrow cells via modulating surface expression of CXCR4
Abstract
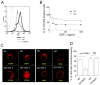
Missing in metastasis (MIM) is abundantly expressed in hematopoietic cells. Here we characterized the impact of MIM deficiency on murine bone marrow (BM) cells. Although MIM(-/-) cells proliferated similarly to wild type (WT), they exhibited stronger response to chemokine stromal-derived factor 1 (SDF-1), increase in surface expression of CXCR4, impaired CXCR4 internalization and constitutive activation of Rac, Cdc42 and p38. Transplantation of MIM(-/-) BM cells into lethally irradiated mice showed enhanced homing to BM, which was abolished when mice were pretreated with a p38 antagonist. Interestingly, MIM(-/-) BM cells, including hematopoietic stem and progenitor cells (HSPCs), showed two- to fivefold increase in mobilization into the peripheral blood upon treatment with AMD3100. In vitro, MIM(-/-) leukocytes were susceptible to AMD3100 and maintained increased response to AMD3100 for mobilization even after transfer into WT mice. MIM(-/-) mice had also a higher level of SDF-1 in the circulation. Our data highlighted an unprecedented role of MIM in the homeostasis of BM cells, including HSPCs, through modulation of the CXCR4/SDF-1 axis and interactions of BM leukocytes with their microenvironments.
Figures

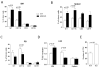
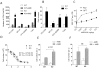


References
-
- Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999 Apr;10(4):463–471. - PubMed
-
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998 Jun 11;393(6685):595–599. - PubMed
-
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006 Dec;25(6):977–988. - PubMed
-
- Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003 Nov 6;22(50):8093–8101. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

