A clinically relevant pharmacokinetic interaction between cyclosporine and imatinib
- PMID: 26965514
- PMCID: PMC4865545
- DOI: 10.1007/s00228-016-2038-9
A clinically relevant pharmacokinetic interaction between cyclosporine and imatinib
Abstract
Purpose: Cyclosporine A (CsA) and imatinib are both CYP3A4 and P-glycoprotein substrates. Concomitant use after hematopoietic stem cell transplantation (HSCT) for chronic myeloid leukemia (CML) or Philadelphia chromosome-positive (Ph+) acute lymphatic leukemia (ALL) may therefore result in a pharmacokinetic interaction. Although case reports and a recent small study in children indeed suggested there is a relevant pharmacokinetic interaction, a larger study in adults is lacking. In this study, we assessed the presence and extent of this interaction in patients with CML or Ph+ ALL undergoing HSCT.
Methods: From a large database containing data of all patients receiving HSCT in our center between 2005 and 2015, we selected 16 patients using this drug combination. The average dose-corrected CsA concentration was calculated before and after initiation of imatinib.
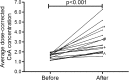
Results: The average dose-corrected CsA concentration increased during imatinib use in all patients, on average by 94 % (p < 0.001). Based on measured drug concentrations, the CsA dosage needed to be reduced, on average, by 27 % after initiation of imatinib (p = 0.004).
Conclusions: Imatinib significantly increases CsA concentrations in HSCT patients, putting these patients at increased risk of CsA toxicity. We recommend intensive monitoring of CsA concentrations after initiation of imatinib; a pre-emptive CsA dose reduction of 25 % might be considered.
Keywords: Cyclosporine; Graft-versus-host disease; Hematopoietic stem cell transplantation; Imatinib.
Figures
Similar articles
-
Co-administration of cyclosporine A and imatinib among patients with Philadelphia chromosome-positive leukemias in the post-transplant setting.Eur J Clin Pharmacol. 2016 Dec;72(12):1537-1538. doi: 10.1007/s00228-016-2122-1. Epub 2016 Aug 25. Eur J Clin Pharmacol. 2016. PMID: 27558360 No abstract available.
-
Imatinib Mesylate Versus Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Chronic Myelogenous Leukemia.Asian Pac J Cancer Prev. 2016;17(9):4477-4481. Asian Pac J Cancer Prev. 2016. PMID: 27797264
-
Pharmacokinetic drug interaction between cyclosporine and imatinib in bone marrow transplant children and model-based reappraisal of imatinib drug interaction profile.Ther Drug Monit. 2014 Dec;36(6):724-9. doi: 10.1097/FTD.0000000000000084. Ther Drug Monit. 2014. PMID: 24739665
-
Imatinib mesylate in chronic myeloid leukemia: frontline treatment and long-term outcomes.Expert Rev Anticancer Ther. 2016;16(3):273-8. doi: 10.1586/14737140.2016.1151356. Expert Rev Anticancer Ther. 2016. PMID: 26852913 Review.
-
Pharmacology and pharmacokinetics of imatinib in pediatric patients.Expert Rev Clin Pharmacol. 2018 Mar;11(3):219-231. doi: 10.1080/17512433.2018.1398644. Epub 2017 Nov 6. Expert Rev Clin Pharmacol. 2018. PMID: 29076384 Review.
Cited by
-
Co-administration of cyclosporine A and imatinib among patients with Philadelphia chromosome-positive leukemias in the post-transplant setting.Eur J Clin Pharmacol. 2016 Dec;72(12):1537-1538. doi: 10.1007/s00228-016-2122-1. Epub 2016 Aug 25. Eur J Clin Pharmacol. 2016. PMID: 27558360 No abstract available.
-
Real-world efficacy and safety outcomes of imatinib treatment in patients with chronic myeloid leukemia: An Australian experience.Pharmacol Res Perspect. 2022 Oct;10(5):e01005. doi: 10.1002/prp2.1005. Pharmacol Res Perspect. 2022. PMID: 36106342 Free PMC article.
-
A Single Dose of Baicalin Has No Clinically Significant Effect on the Pharmacokinetics of Cyclosporine A in Healthy Chinese Volunteers.Front Pharmacol. 2019 May 14;10:518. doi: 10.3389/fphar.2019.00518. eCollection 2019. Front Pharmacol. 2019. PMID: 31156436 Free PMC article.
-
Molecular Mechanisms of Tyrosine Kinase Inhibitor Resistance in Chronic Myeloid Leukemia.Handb Exp Pharmacol. 2023;280:65-83. doi: 10.1007/164_2023_639. Handb Exp Pharmacol. 2023. PMID: 36882601 Review.
-
Converting cyclosporine A from intravenous to oral administration in hematopoietic stem cell transplant recipients and the role of azole antifungals.Eur J Clin Pharmacol. 2018 Jun;74(6):767-773. doi: 10.1007/s00228-018-2434-4. Epub 2018 Mar 2. Eur J Clin Pharmacol. 2018. PMID: 29500599 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

