CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils
- PMID: 26965633
- PMCID: PMC6608091
- DOI: 10.1189/jlb.3A1015-480R
CD63 is tightly associated with intracellular, secretory events chaperoning piecemeal degranulation and compound exocytosis in human eosinophils
Abstract
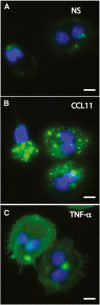
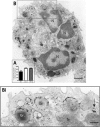
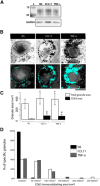
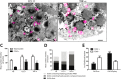
Eosinophil activation leads to secretion of presynthesized, granule-stored mediators that determine the course of allergic, inflammatory, and immunoregulatory responses. CD63, a member of the transmembrane-4 glycoprotein superfamily (tetraspanins) and present on the limiting membranes of eosinophil-specific (secretory) granules, is considered a potential surface marker for eosinophil degranulation. However, the intracellular secretory trafficking of CD63 in eosinophils and other leukocytes is not understood. Here, we provide a comprehensive investigation of CD63 trafficking at high resolution within human eosinophils stimulated with inflammatory stimuli, CCL11 and tumor necrosis factor α, which induce distinctly differing secretory processes in eosinophils: piecemeal degranulation and compound exocytosis, respectively. By using different transmission electron microscopy approaches, including an immunonanogold technique, for enhanced detection of CD63 at subcellular compartments, we identified a major intracellular pool of CD63 that is directly linked to eosinophil degranulation events. Transmission electron microscopy quantitative analyses demonstrated that, in response to stimulation, CD63 is concentrated within granules undergoing secretion by piecemeal degranulation or compound exocytosis and that CD63 tracks with the movements of vesicles and granules in the cytoplasm. Although CD63 was observed at the cell surface after stimulation, immunonanogold electron microscopy revealed that a strong CD63 pool remains in the cytoplasm. It is remarkable that CCL11 and tumor necrosis factor α triggered increased formation of CD63(+) large vesiculotubular carriers (eosinophil sombrero vesicles), which fused with granules in the process of secretion, likely acting in the intracellular translocation of CD63. Altogether, we identified active, intracellular CD63 trafficking connected to eosinophil granule-derived secretory pathways. This is important for understanding the complex secretory activities of eosinophils underlying immune responses.
Keywords: cell secretion; immune responses; inflammation; transmission electron microscopy; vesicular trafficking.
© Society for Leukocyte Biology.
Figures



References
-
- Stow, J. L. , Murray, R. Z. (2013) Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 24, 227–239. - PubMed
-
- Melo, R. C. N. , Dvorak, A. M. , Weller, P. F. 2012. Eosinophil ultrastructure In Eosinophils in health and disease. (Lee J. R., Rosenberg H., eds.), Vol. 1 Elsevier, New York, p. 20–27.
-
- Inoue, Y. , Matsuwaki, Y. , Shin, S. H. , Ponikau, J. U. , Kita, H. (2005) Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J. Immunol. 175, 5439–5447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

