The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells
- PMID: 26965643
- PMCID: PMC4786801
- DOI: 10.1038/srep22966
The Wnt/β-catenin signaling/Id2 cascade mediates the effects of hypoxia on the hierarchy of colorectal-cancer stem cells
Abstract
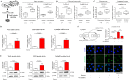
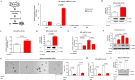
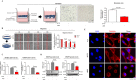
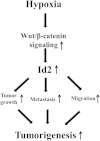
Hypoxia, a feature common to most solid tumors, is known to regulate many aspects of tumorigenesis. Recently, it was suggested that hypoxia increased the size of the cancer stem-cell (CSC) subpopulations and promoted the acquisition of a CSC-like phenotype. However, candidate hypoxia-regulated mediators specifically relevant to the stemness-related functions of colorectal CSCs have not been examined in detail. In the present study, we showed that hypoxia specifically promoted the self-renewal potential of CSCs. Through various in vitro studies, we found that hypoxia-induced Wnt/β-catenin signaling increased the occurrence of CSC-like phenotypes and the level of Id2 expression in colorectal-cancer cells. Importantly, the levels of hypoxia-induced CSC-sphere formation and Id2 expression were successfully attenuated by treatment with a Wnt/β-catenin-signaling inhibitor. We further demonstrated, for the first time, that the degree of hypoxia-induced CSC-sphere formation (CD44(+) subpopulation) in vitro and of tumor metastasis/dissemination in vivo were markedly suppressed by knocking down Id2 expression. Taken together, these data suggested that Wnt/β-catenin signaling mediated the hypoxia-induced self-renewal potential of colorectal-cancer CSCs through reactivating Id2 expression.
Figures



References
-
- Vaupel P. Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist 13 Suppl 3, 21–6 (2008). - PubMed
-
- Visvader J. E. & Lindeman G. J. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10, 717–28 (2012). - PubMed
-
- Li X. et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100, 672–9 (2008). - PubMed
-
- Bao S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–60 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

