Evidence of Stereoscopic Surface Disambiguation in the Responses of V1 Neurons
- PMID: 26965904
- PMCID: PMC6317328
- DOI: 10.1093/cercor/bhw064
Evidence of Stereoscopic Surface Disambiguation in the Responses of V1 Neurons
Abstract
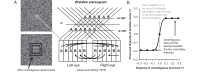
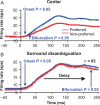
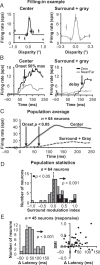
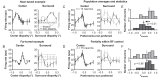
For the important task of binocular depth perception from complex natural-image stimuli, the neurophysiological basis for disambiguating multiple matches between the eyes across similar features has remained a long-standing problem. Recurrent interactions among binocular disparity-tuned neurons in the primary visual cortex (V1) could play a role in stereoscopic computations by altering responses to favor the most likely depth interpretation for a given image pair. Psychophysical research has shown that binocular disparity stimuli displayed in 1 region of the visual field can be extrapolated into neighboring regions that contain ambiguous depth information. We tested whether neurons in macaque V1 interact in a similar manner and found that unambiguous binocular disparity stimuli displayed in the surrounding visual fields of disparity-selective V1 neurons indeed modified their responses when either bistable stereoscopic or uniform featureless stimuli were presented within their receptive field centers. The delayed timing of the response behavior compared with the timing of classical surround suppression and multiple control experiments suggests that these modulations are carried out by slower disparity-specific recurrent connections among V1 neurons. These results provide explicit evidence that the spatial interactions that are predicted by cooperative algorithms play an important role in solving the stereo correspondence problem.
Keywords: macaque; primary visual cortex; recurrent; stereoscopic; surround.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures





Similar articles
-
Local disparity not perceived depth is signaled by binocular neurons in cortical area V1 of the Macaque.J Neurosci. 2000 Jun 15;20(12):4758-67. doi: 10.1523/JNEUROSCI.20-12-04758.2000. J Neurosci. 2000. PMID: 10844045 Free PMC article.
-
Responses of primary visual cortical neurons to binocular disparity without depth perception.Nature. 1997 Sep 18;389(6648):280-3. doi: 10.1038/38487. Nature. 1997. PMID: 9305841
-
Disparity Sensitivity and Binocular Integration in Mouse Visual Cortex Areas.J Neurosci. 2020 Nov 11;40(46):8883-8899. doi: 10.1523/JNEUROSCI.1060-20.2020. Epub 2020 Oct 13. J Neurosci. 2020. PMID: 33051348 Free PMC article.
-
Mechanisms of stereopsis in monkey visual cortex.Cereb Cortex. 1995 May-Jun;5(3):193-204. doi: 10.1093/cercor/5.3.193. Cereb Cortex. 1995. PMID: 7613075 Review.
-
Early computational processing in binocular vision and depth perception.Prog Biophys Mol Biol. 2005 Jan;87(1):77-108. doi: 10.1016/j.pbiomolbio.2004.06.005. Prog Biophys Mol Biol. 2005. PMID: 15471592 Free PMC article. Review.
Cited by
-
A novel dynamic random-dot stereopsis assessment to measure stereopsis in intermittent exotropia.Ann Transl Med. 2021 Feb;9(4):308. doi: 10.21037/atm-20-3896. Ann Transl Med. 2021. PMID: 33708935 Free PMC article.
-
Relating functional connectivity in V1 neural circuits and 3D natural scenes using Boltzmann machines.Vision Res. 2016 Mar;120:121-31. doi: 10.1016/j.visres.2015.12.002. Epub 2015 Dec 28. Vision Res. 2016. PMID: 26712581 Free PMC article.
-
Contextual Binocular Imbalance Impairs Local Stereopsis.Invest Ophthalmol Vis Sci. 2025 Mar 3;66(3):6. doi: 10.1167/iovs.66.3.6. Invest Ophthalmol Vis Sci. 2025. PMID: 40035726 Free PMC article.
-
Mice Discriminate Stereoscopic Surfaces Without Fixating in Depth.J Neurosci. 2019 Oct 9;39(41):8024-8037. doi: 10.1523/JNEUROSCI.0895-19.2019. Epub 2019 Aug 28. J Neurosci. 2019. PMID: 31462533 Free PMC article.
-
Sample skewness as a statistical measurement of neuronal tuning sharpness.Neural Comput. 2014 May;26(5):860-906. doi: 10.1162/NECO_a_00582. Epub 2014 Feb 20. Neural Comput. 2014. PMID: 24555451 Free PMC article.
References
-
- Allman J, Miezin F, McGuinness E. 1985. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local-global comparisons in visual neurons. Annu Rev Neurosci. 8:407–430. - PubMed
-
- Angelucci A, Bullier J. 2003. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? J Physiol Paris. 97:141–154. - PubMed
-
- Bauer R, Heinze S. 2002. Contour integration in striate cortex. Exp Brain Res. 147:145–152. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

