Comparative genomics analysis of mononuclear phagocyte subsets confirms homology between lymphoid tissue-resident and dermal XCR1(+) DCs in mouse and human and distinguishes them from Langerhans cells
- PMID: 26966045
- PMCID: PMC4859332
- DOI: 10.1016/j.jim.2016.02.023
Comparative genomics analysis of mononuclear phagocyte subsets confirms homology between lymphoid tissue-resident and dermal XCR1(+) DCs in mouse and human and distinguishes them from Langerhans cells
Abstract
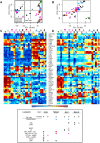
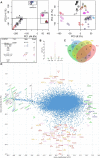
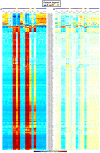
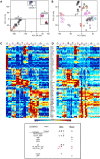
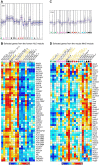
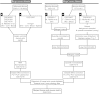
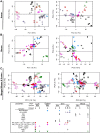
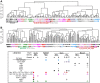
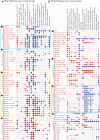
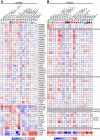
Dendritic cells (DC) are mononuclear phagocytes which exhibit a branching (dendritic) morphology and excel at naïve T cell activation. DC encompass several subsets initially identified by their expression of cell surface molecules and later shown to possess distinct functions. DC subset differentiation is orchestrated by transcription factors, growth factors and cytokines. Identifying DC subsets is challenging as very few cell surface molecules are uniquely expressed on any one of these cell populations. There is no standard consensus to identify mononuclear phagocyte subsets; varying antigens are employed depending on the tissue and animal species studied and between laboratories. This has led to confusion in how to accurately define and classify DCs across tissues and between species. Here we report a comparative genomics strategy that enables universal definition of DC and other mononuclear phagocyte subsets across species. We performed a meta-analysis of several public datasets of human and mouse mononuclear phagocyte subsets isolated from blood, spleen, skin or cutaneous lymph nodes, including by using a novel and user friendly software, BubbleGUM, which generates and integrates gene signatures for high throughput gene set enrichment analysis. This analysis demonstrates the equivalence between human and mouse skin XCR1(+) DCs, and between mouse and human Langerhans cells.
Keywords: Bioinformatics; Comparative genomics; Dendritic cells; Langerhans cells; Skin; XCR1.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures








References
-
- Balan S., Ollion V., Colletti N., Chelbi R., Montanana-Sanchis F., Liu H., Vu Manh T.P., Sanchez C., Savoret J., Perrot I. Human XCR1 + dendritic cells derived in vitro from CD34 + progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells. J. Immunol. 2014;193:1622–1635. - PMC - PubMed
-
- Becher B., Schlitzer A., Chen J., Mair F., Sumatoh H.R., Teng K.W., Low D., Ruedl C., Riccardi-Castagnoli P., Poidinger M. High-dimensional analysis of the murine myeloid cell system. Nat. Immunol. 2014;15:1181–1189. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

