Epigenetic Mechanisms of the Aging Human Retina
- PMID: 26966390
- PMCID: PMC4777243
- DOI: 10.4137/JEN.S25513
Epigenetic Mechanisms of the Aging Human Retina
Abstract
Degenerative retinal diseases, such as glaucoma, age-related macular degeneration, and diabetic retinopathy, have complex etiologies with environmental, genetic, and epigenetic contributions to disease pathology. Much effort has gone into elucidating both the genetic and the environmental risk factors for these retinal diseases. However, little is known about how these genetic and environmental risk factors bring about molecular changes that lead to pathology. Epigenetic mechanisms have received extensive attention of late for their promise of bridging the gap between environmental exposures and disease development via their influence on gene expression. Recent studies have identified epigenetic changes that associate with the incidence and/or progression of each of these retinal diseases. Therefore, these epigenetic modifications may be involved in the underlying pathological mechanisms leading to blindness. Further genome-wide epigenetic studies that incorporate well-characterized tissue samples, consider challenges similar to those relevant to gene expression studies, and combine the genome-wide epigenetic data with genome-wide genetic and expression data to identify additional potentially causative agents of disease are needed. Such studies will allow researchers to create much-needed therapeutics to prevent and/or intervene in disease progression. Improved therapeutics will greatly enhance the quality of life and reduce the burden of disease management for millions of patients living with these potentially blinding conditions.
Keywords: DNA methylation; age-related macular degeneration; aging; chromatin; diabetic retinopathy; epigenetics; genome-wide; glaucoma; histone modification; neurodegeneration.
Figures


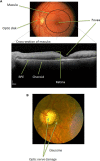

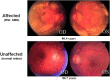
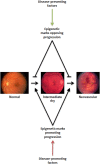

References
-
- Piña B, Brüggemeier U, M Beato. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990;60(5):719–731. - PubMed
-
- Gregory PD, Hörz W. Chromatin and transcription—how transcription factors battle with a repressive chromatin environment. Eur J Biochem. 1998;251:1–2. 9–18. - PubMed
-
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. - PubMed
-
- Wozniak GG, Strahl BD. Hitting the “mark”: interpreting lysine methylation in the context of active transcription. Biochim Biophys Acta. 2014;1839(12):1353–1361. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

