Standardizing Umbilical Cord Mesenchymal Stromal Cells for Translation to Clinical Use: Selection of GMP-Compliant Medium and a Simplified Isolation Method
- PMID: 26966439
- PMCID: PMC4757747
- DOI: 10.1155/2016/6810980
Standardizing Umbilical Cord Mesenchymal Stromal Cells for Translation to Clinical Use: Selection of GMP-Compliant Medium and a Simplified Isolation Method
Abstract
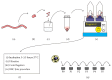
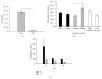
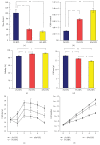
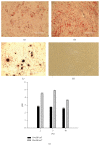
Umbilical cord derived mesenchymal stromal cells (UC-MSCs) are a focus for clinical translation but standardized methods for isolation and expansion are lacking. Previously we published isolation and expansion methods for UC-MSCs which presented challenges when considering good manufacturing practices (GMP) for clinical translation. Here, a new and more standardized method for isolation and expansion of UC-MSCs is described. The new method eliminates dissection of blood vessels and uses a closed-vessel dissociation following enzymatic digestion which reduces contamination risk and manipulation time. The new method produced >10 times more cells per cm of UC than our previous method. When biographical variables were compared, more UC-MSCs per gram were isolated after vaginal birth compared to Caesarian-section births, an unexpected result. UC-MSCs were expanded in medium enriched with 2%, 5%, or 10% pooled human platelet lysate (HPL) eliminating the xenogeneic serum components. When the HPL concentrations were compared, media supplemented with 10% HPL had the highest growth rate, smallest cells, and the most viable cells at passage. UC-MSCs grown in 10% HPL had surface marker expression typical of MSCs, high colony forming efficiency, and could undergo trilineage differentiation. The new protocol standardizes manufacturing of UC-MSCs and enables clinical translation.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

