Profiles of direct oral anticoagulants and clinical usage-dosage and dose regimen differences
- PMID: 26966542
- PMCID: PMC4785699
- DOI: 10.1186/s40560-016-0144-5
Profiles of direct oral anticoagulants and clinical usage-dosage and dose regimen differences
Abstract
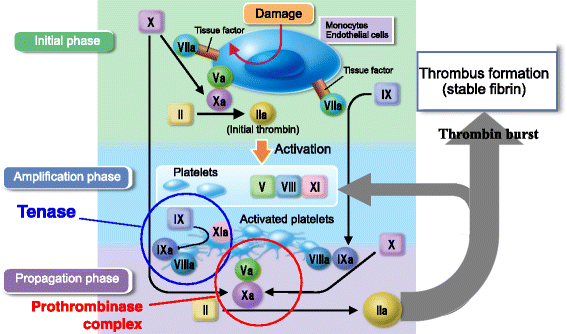
The availability of direct oral anticoagulants (DOACs) has caused a paradigm shift in thrombosis management. DOAC profiles do not differ greatly, though they are quite different from that of warfarin, whereas their dosage and dose regimens are not consistent. The direct thrombin inhibitor dabigatran seems to obstruct tenase by inhibiting thrombin generated in the initial phase and feedback to the amplification phase of cell-based coagulation reactions. Factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) mainly inhibit factor Xa activity of the prothrombinase complex in the propagation phase. The dose regimens of these inhibitors can be classified into once (rivaroxaban, edoxaban) and twice (dabigatran, apixaban) daily. On the other hand, their plasma elimination half-life times are similar, which can be explained by differences in the type of aimed anticoagulation, such as persistent (e.g., warfarin) and intermittent (e.g., low-molecular-weight heparin). Because of the differences among DOACs, an indicator is necessary to compare them. We investigated relative potency to compare dosage and intensity by calculation of conversion using a profile comprised of molecular weight, bioavailability, protein-binding rate, inhibitory constant, and dosage. We found that the relative potencies were different, with that of apixaban higher than edoxaban (60 mg) and nearly twice that of rivaroxaban. However, dabigatran could not be evaluated with this profile, likely due to its different mode of action. These results suggest that rivaroxaban and apixaban differ in regard to anticoagulation type, as the former shows persistent and the latter intermittent anticoagulation.
Keywords: Anticoagulant therapy; Direct oral anticoagulant (DOAC); Relative potency; Stroke prevention in atrial fibrillation (SPAF); Warfarin.
Figures

References
-
- Hoffman M, Monroe DM, Roberts HR. Cellular interaction in hemostasis. Haemostasis. 1996;26(Supple):12–6. - PubMed
-
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, Vandij CN, Frostick SP, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–85. doi: 10.1111/j.1538-7836.2007.02748.x. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

