Oral Mineralocorticoid-Receptor Antagonists: Real-Life Experience in Clinical Subtypes of Nonresolving Central Serous Chorioretinopathy With Chronic Epitheliopathy
- PMID: 26966638
- PMCID: PMC4782825
- DOI: 10.1167/tvst.5.2.2
Oral Mineralocorticoid-Receptor Antagonists: Real-Life Experience in Clinical Subtypes of Nonresolving Central Serous Chorioretinopathy With Chronic Epitheliopathy
Abstract
Purpose: To evaluate the efficacy and safety of oral mineralocorticoid-receptor antagonist (MRa) therapy in three clinical presentations of nonresolving central serous chorioretinopathy (CSCR) with chronic epitheliopathy.
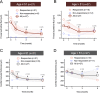
Methods: Retrospective case series of consecutive patients with nonresolving CSCR treated with oral eplerenone or spironolactone. Treatment criteria were: persistent CSCR with subretinal fluid (SRF) lasting longer than 4 months; recurrent CSCR with SRF lasting longer than 2 months; persistent CSCR (SRF ≥ 4 months) with fundus autofluorescence gravitational tracks. Outcomes at 1, 3, and 6 months were: foveal SRF height, central macular thickness (CMT), subfoveal choroidal thickness (SFCT), best-corrected visual acuity (BCVA), and occurrence of side effects.
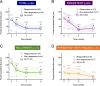
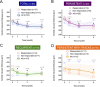
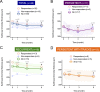
Results: Among 54 eyes from 42 patients (mean age: 53 years), mean foveal SRF, CMT, and SFCT decreased significantly at 1, 3, and 6 months after treatment initiation. Mean BCVA improved significantly at 6 months. In the subgroup analysis, mean foveal SRF, CMT, and SFCT decreased significantly at 3 and 6 months in the persistent and recurrent groups. In persistent cases with tracks, a significant diminution of mean CMT and SFCT was achieved at 6 months. Treatment-related side effects were observed in 6 patients, prompting treatment discontinuation in one case.
Conclusion: Response to treatment was observed in the three subgroups. In persistent CSCR with tracks the response was delayed compared with persistent and recurrent cases, suggesting that longer treatment durations would be beneficial in patients with gravitational tracks of RPE alteration.
Translational relevance: The clinical response to oral MRa is consistent with the involvement of the mineralocorticoid pathway in CSCR pathogenesis.
Keywords: central serous chorioretinopathy; choroid; drug-related side effects and adverse reactions; mineralocorticoid-receptor antagonist; retinal pigment epithelium; spironolactone; treatment, epleronone.
Figures

References
-
- Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010; 149: 361–363. - PubMed
-
- Yannuzzi LA,, Slakter JS,, Kaufman SR,, Gupta K. Laser treatment of diffuse retinal pigment epitheliopathy. Eur J Ophthalmol. 1992; 2: 103–114. - PubMed
-
- Piccolino FC,, De La Longrais RR,, Manea M,, Cicinelli S. Posterior cystoid retinal degeneration in central serous chorioretinopathy. Retina. 2008; 28: 1008–1012. - PubMed
-
- Ooto S,, Hangai M,, Sakamoto A,, et al. High-resolution imaging of resolved central serous chorioretinopathy using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2010; 117: 1800–1809. - PubMed
-
- Piccolino FC,, de la Longrais RR,, Ravera G,, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005; 139: 87–99. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

