Enantiodivergent Fluorination of Allylic Alcohols: Data Set Design Reveals Structural Interplay between Achiral Directing Group and Chiral Anion
- PMID: 26967114
- PMCID: PMC5176255
- DOI: 10.1021/jacs.6b00356
Enantiodivergent Fluorination of Allylic Alcohols: Data Set Design Reveals Structural Interplay between Achiral Directing Group and Chiral Anion
Abstract
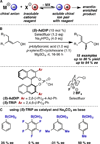
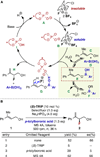
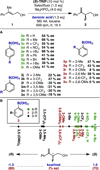
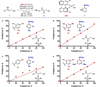
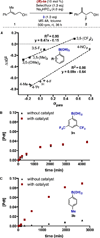
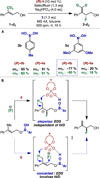
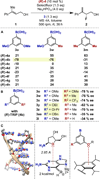
Enantioselectivity values represent relative rate measurements that are sensitive to the structural features of the substrates and catalysts interacting to produce them. Therefore, well-designed enantioselectivity data sets are information rich and can provide key insights regarding specific molecular interactions. However, if the mechanism for enantioselection varies throughout a data set, these values cannot be easily compared. This premise, which is the crux of free energy relationships, exposes a challenging issue of identifying mechanistic breaks within multivariate correlations. Herein, we describe an approach to addressing this problem in the context of a chiral phosphoric acid catalyzed fluorination of allylic alcohols using aryl boronic acids as transient directing groups. By designing a data set in which both the phosphoric and boronic acid structures were systematically varied, key enantioselectivity outliers were identified and analyzed. A mechanistic study was executed to reveal the structural origins of these outliers, which was consistent with the presence of several mechanistic regimes within the data set. While 2- and 4-substituted aryl boronic acids favored the (R)-enantiomer with most of the studied catalysts, meta-alkoxy substituted aryl boronic acids resulted in the (S)-enantiomer when used in combination with certain (R)-phosphoric acids. We propose that this selectivity reversal is the result of a lone pair-π interaction between the substrate ligated boronic acid and the phosphate. On the basis of this proposal, a catalyst system was identified, capable of producing either enantiomer in high enantioselectivity (77% (R)-2 to 92% (S)-2) using the same chiral catalyst by subtly changing the structure of the achiral boronic acid.
Figures



Similar articles
-
An in situ directing group strategy for chiral anion phase-transfer fluorination of allylic alcohols.J Am Chem Soc. 2014 Sep 17;136(37):12864-7. doi: 10.1021/ja507468u. Epub 2014 Sep 9. J Am Chem Soc. 2014. PMID: 25203796 Free PMC article.
-
Enantioselective epoxidation with chiral MN(III)(salen) catalysts: kinetic resolution of aryl-substituted allylic alcohols.J Org Chem. 2001 Aug 24;66(17):5796-800. doi: 10.1021/jo010350j. J Org Chem. 2001. PMID: 11511254
-
Enantioselective fluorination of homoallylic alcohols enabled by the tuning of non-covalent interactions.Chem Sci. 2018 Aug 3;9(35):7153-7158. doi: 10.1039/c8sc02223b. eCollection 2018 Sep 21. Chem Sci. 2018. PMID: 30310638 Free PMC article.
-
Homologation and alkylation of boronic esters and boranes by 1,2-metallate rearrangement of boronate complexes.Chem Rec. 2009;9(1):24-39. doi: 10.1002/tcr.20168. Chem Rec. 2009. PMID: 19243084 Review.
-
Catalytic asymmetric organozinc additions to carbonyl compounds.Chem Rev. 2001 Mar;101(3):757-824. doi: 10.1021/cr000411y. Chem Rev. 2001. PMID: 11712502 Review.
Cited by
-
Enantioselective Kinetic Resolution/Desymmetrization of Para-Quinols: A Case Study in Boronic-Acid-Directed Phosphoric Acid Catalysis.Adv Synth Catal. 2020 Jan 23;362(2):295-301. doi: 10.1002/adsc.201900816. Epub 2019 Sep 6. Adv Synth Catal. 2020. PMID: 34093103 Free PMC article.
-
Brønsted acid catalysis - the effect of 3,3'-substituents on the structural space and the stabilization of imine/phosphoric acid complexes.Chem Sci. 2019 Apr 8;10(20):5226-5234. doi: 10.1039/c9sc01044k. eCollection 2019 May 28. Chem Sci. 2019. PMID: 31191877 Free PMC article.
-
Catalysing (organo-)catalysis: Trends in the application of machine learning to enantioselective organocatalysis.Beilstein J Org Chem. 2024 Sep 10;20:2280-2304. doi: 10.3762/bjoc.20.196. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39290209 Free PMC article. Review.
-
Reaction performance prediction with an extrapolative and interpretable graph model based on chemical knowledge.Nat Commun. 2023 Jun 15;14(1):3569. doi: 10.1038/s41467-023-39283-x. Nat Commun. 2023. PMID: 37322041 Free PMC article.
-
Development and Analysis of a Pd(0)-Catalyzed Enantioselective 1,1-Diarylation of Acrylates Enabled by Chiral Anion Phase Transfer.J Am Chem Soc. 2016 Dec 14;138(49):15877-15880. doi: 10.1021/jacs.6b11367. Epub 2016 Nov 30. J Am Chem Soc. 2016. PMID: 27960315 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

