Pharmacological Modulation of Hemodynamics in Adult Zebrafish In Vivo
- PMID: 26967155
- PMCID: PMC4788458
- DOI: 10.1371/journal.pone.0150948
Pharmacological Modulation of Hemodynamics in Adult Zebrafish In Vivo
Abstract
Introduction: Hemodynamic parameters in zebrafish receive increasing attention because of their important role in cardiovascular processes such as atherosclerosis, hematopoiesis, sprouting and intussusceptive angiogenesis. To study underlying mechanisms, the precise modulation of parameters like blood flow velocity or shear stress is centrally important. Questions related to blood flow have been addressed in the past in either embryonic or ex vivo-zebrafish models but little information is available for adult animals. Here we describe a pharmacological approach to modulate cardiac and hemodynamic parameters in adult zebrafish in vivo.
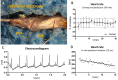
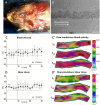
Materials and methods: Adult zebrafish were paralyzed and orally perfused with salt water. The drugs isoprenaline and sodium nitroprusside were directly applied with the perfusate, thus closely resembling the preferred method for drug delivery in zebrafish, namely within the water. Drug effects on the heart and on blood flow in the submental vein were studied using electrocardiograms, in vivo-microscopy and mathematical flow simulations.
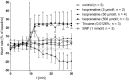
Results: Under control conditions, heart rate, blood flow velocity and shear stress varied less than ± 5%. Maximal chronotropic effects of isoprenaline were achieved at a concentration of 50 μmol/L, where it increased the heart rate by 22.6 ± 1.3% (n = 4; p < 0.0001). Blood flow velocity and shear stress in the submental vein were not significantly increased. Sodium nitroprusside at 1 mmol/L did not alter the heart rate but increased blood flow velocity by 110.46 ± 19.64% (p = 0.01) and shear stress by 117.96 ± 23.65% (n = 9; p = 0.03).
Discussion: In this study, we demonstrate that cardiac and hemodynamic parameters in adult zebrafish can be efficiently modulated by isoprenaline and sodium nitroprusside. Together with the suitability of the zebrafish for in vivo-microscopy and genetic modifications, the methodology described permits studying biological processes that are dependent on hemodynamic alterations.
Conflict of interest statement
Figures
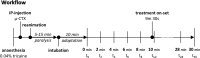

References
-
- Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8: 353–367. - PubMed
-
- Poss KD, Keating MT, Nechiporuk A (2003) Tales of regeneration in zebrafish. Dev Dyn 226: 202–210. - PubMed
-
- Iovine MK (2007) Conserved mechanisms regulate outgrowth in zebrafish fins. NatChemBiol 3: 613–618. - PubMed
-
- Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S (1983) Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 53: 502–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

