Generation and Characterization of a Human/Mouse Chimeric GD2-Mimicking Anti-Idiotype Antibody Ganglidiximab for Active Immunotherapy against Neuroblastoma
- PMID: 26967324
- PMCID: PMC4788445
- DOI: 10.1371/journal.pone.0150479
Generation and Characterization of a Human/Mouse Chimeric GD2-Mimicking Anti-Idiotype Antibody Ganglidiximab for Active Immunotherapy against Neuroblastoma
Abstract
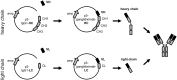
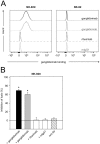
Vaccination with proteins mimicking GD2 that is highly expressed on neuroblastoma (NB) cells is a promising strategy in treatment of NB, a pediatric malignancy with poor prognosis. We previously showed efficacy of ganglidiomab in vivo, a murine anti-idiotype (anti-Id) IgG1. In order to tailor immune responses to variable regions, we generated a new human/mouse chimeric anti-Id antibody (Ab) ganglidiximab by replacing murine constant fragments with corresponding human IgG1 regions. DNA sequences encoding for variable regions of heavy (VH) and light chains (VL) were synthesized by RT-PCR from total RNA of ganglidiomab-producing hybridoma cells and further ligated into mammalian expression plasmids with coding sequences for constant regions of human IgG1 heavy and light chains, respectively. We established a stable production cell line using Chinese hamster ovarian (CHO) cells co-transfected with two expression plasmids driving the expression of either ganglidiximab heavy or light chain. After purification from supernatants, anti-idiotypic characteristics of ganglidiximab were demonstrated. Binding of ganglidiximab to anti-GD2 Abs of the 14.18 family as well as to NK-92tr cells expressing a GD2-specific chimeric antigen receptor (scFv(ch14.18)-zeta) was shown using standard ELISA and flow cytometry analysis, respectively. Ganglidiximab binding affinities to anti-GD2 Abs were further determined by surface plasmon resonance technique. Moreover, binding of anti-GD2 Abs to the nominal antigen GD2 as well as GD2-specific Ab-mediated cytotoxicity (ADCC, CDC) was competitively inhibited by ganglidiximab. Finally, ganglidiximab was successfully used as a protein vaccine in vivo to induce a GD2-specific humoral immune response. In summary, we report generation and characterization of a new human/mouse chimeric anti-Id Ab ganglidiximab for active immunotherapy against NB. This Ab may be useful to tailor immune responses to the paratope regions mimicking GD2 overexpressed in NB.
Conflict of interest statement
Figures





References
-
- Alice L.Yu. Progress in Treatment of High-risk Neuroblastoma with Immunotherapy. American Society of Clinical Oncology, American Society of Clinical Oncology.
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007. June 23;369(9579):2106–20. - PubMed
-
- Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J Clin Oncol. 2009. March 1;27(7):1007–13. 10.1200/JCO.2007.13.8925 - DOI - PMC - PubMed
-
- Cheung NK, Neely JE, Landmeier B, Nelson D, Miraldi F. Targeting of ganglioside GD2 monoclonal antibody to neuroblastoma. J Nucl Med. 1987. October;28(10):1577–83. - PubMed
-
- Schulz G, Cheresh DA, Varki NM, Yu A, Staffileno LK, Reisfeld RA. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 1984. December;44(12 Pt 1):5914–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

