A systematic evaluation of the safety and toxicity of fingolimod for its potential use in the treatment of acute myeloid leukaemia
- PMID: 26967515
- PMCID: PMC4881728
- DOI: 10.1097/CAD.0000000000000358
A systematic evaluation of the safety and toxicity of fingolimod for its potential use in the treatment of acute myeloid leukaemia
Abstract
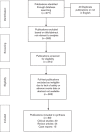
Treatment of acute myeloid leukaemia (AML) is challenging and emerging treatment options include protein phosphatase 2A (PP2A) activators. Fingolimod is a known PP2A activator that inhibits multiple signalling pathways and has been used extensively in patients with multiple sclerosis and other indications. The initial positive results of PP2A activators in vitro and mouse models of AML are promising; however, its safety for use in AML has not been assessed. From human studies of fingolimod in other indications, it is possible to evaluate whether the safety and toxicity profile of the PP2A activators will allow their use in treating AML. A literature review was carried out to assess safety before the commencement of Phase I trials of the PP2A activator Fingolimod in AML. From human studies of fingolimod in other indications, it is possible to evaluate whether the safety and toxicity profile of the PP2A activators will allow their use in treating AML. A systematic review of published literature in Medline, EMBASE and the Cochrane Library of critical reviews was carried out. International standards for the design and reporting of search strategies were followed. Search terms and medical subject headings used in trials involving PP2A activators as well as a specific search were performed for 'adverse events', 'serious adverse events', 'delays in treatment', ' side effects' and 'toxicity' for primary objectives. Database searches were limited to papers published in the last 12 years and available in English. The search yielded 677 articles. A total of 69 journal articles were identified as relevant and included 30 clinical trials, 24 review articles and 15 case reports. The most frequently reported adverse events were nausea, diarrhoea, fatigue, back pain, influenza viral infections, nasopharyngitis and bronchitis. Specific safety concerns include monitoring of the heart rate and conduction at commencement of treatment as cardiotoxicity has been reported. There is little evidence to suggest specific bone marrow toxicity. Lymophopenia is a desired effect in the management of multiple sclerosis, but may have implications in patients with acute leukaemia as it may potentially increase susceptibility to viral infections such as influenza. Fingolimod is a potential treatment option for AML with an acceptable risk to benefit ratio, given its lack of bone marrow toxicity and the relatively low rate of serious side effects. As most patients with AML are elderly, specific monitoring for cardiac toxicity as well as infection would be required.
Figures
Similar articles
-
A novel FTY720 analogue targets SET-PP2A interaction and inhibits growth of acute myeloid leukemia cells without inducing cardiac toxicity.Cancer Lett. 2020 Jan 1;468:1-13. doi: 10.1016/j.canlet.2019.10.007. Epub 2019 Oct 5. Cancer Lett. 2020. PMID: 31593801
-
Development of novel PP2A activators for use in the treatment of acute myeloid leukaemia.Org Biomol Chem. 2016 May 18;14(20):4605-16. doi: 10.1039/c6ob00556j. Org Biomol Chem. 2016. PMID: 27102578
-
Activation of protein phosphatase 2A in FLT3+ acute myeloid leukemia cells enhances the cytotoxicity of FLT3 tyrosine kinase inhibitors.Oncotarget. 2016 Jul 26;7(30):47465-47478. doi: 10.18632/oncotarget.10167. Oncotarget. 2016. PMID: 27329844 Free PMC article.
-
Update on the cardiovascular profile of fingolimod in the therapy of relapsing-remitting multiple sclerosis (MS).Mult Scler Relat Disord. 2016 Jul;8:19-26. doi: 10.1016/j.msard.2016.04.002. Epub 2016 Apr 21. Mult Scler Relat Disord. 2016. PMID: 27456870 Review.
-
Incidence and Risk of Infection Associated With Fingolimod in Patients With Multiple Sclerosis: A Systematic Review and Meta-Analysis of 8,448 Patients From 12 Randomized Controlled Trials.Front Immunol. 2021 Mar 8;12:611711. doi: 10.3389/fimmu.2021.611711. eCollection 2021. Front Immunol. 2021. PMID: 33763062 Free PMC article.
Cited by
-
PP2A-activating Drugs Enhance FLT3 Inhibitor Efficacy through AKT Inhibition-Dependent GSK-3β-Mediated c-Myc and Pim-1 Proteasomal Degradation.Mol Cancer Ther. 2021 Apr;20(4):676-690. doi: 10.1158/1535-7163.MCT-20-0663. Epub 2021 Feb 10. Mol Cancer Ther. 2021. PMID: 33568357 Free PMC article.
-
Promising Molecular Targets in Pharmacological Therapy for Neuronal Damage in Brain Injury.Antioxidants (Basel). 2023 Jan 3;12(1):118. doi: 10.3390/antiox12010118. Antioxidants (Basel). 2023. PMID: 36670980 Free PMC article. Review.
-
Identifying key genes and functionally enriched pathways in acute myeloid leukemia by weighted gene co-expression network analysis.J Appl Genet. 2025 May;66(2):347-362. doi: 10.1007/s13353-024-00881-0. Epub 2024 Jul 9. J Appl Genet. 2025. PMID: 38977582
-
A statistical network pre-processing method to improve relevance and significance of gene lists in microarray gene expression studies.BMC Bioinformatics. 2022 Sep 27;23(Suppl 6):393. doi: 10.1186/s12859-022-04936-z. BMC Bioinformatics. 2022. PMID: 36167506 Free PMC article.
-
Protein phosphatase 2A as a therapeutic target in inflammation and neurodegeneration.Pharmacol Ther. 2019 Sep;201:181-201. doi: 10.1016/j.pharmthera.2019.05.016. Epub 2019 Jun 1. Pharmacol Ther. 2019. PMID: 31158394 Free PMC article. Review.
References
-
- Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE. Medical Research Council Adult Leukemia Working Party. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood 2001; 98:1302–1311. - PubMed
-
- Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature 2007; 445:53–57. - PubMed
-
- Kranias G, Watt LF, Carpenter H, Holst J, Ludowyke R, Strack S, et al. Protein phosphatase 2A carboxymethylation and regulatory B subunits differentially regulate mast cell degranulation. Cell Signal 2010; 22:1882–1890. - PubMed
-
- Sim AT, Ludowyke RI, Verrills NM. Mast cell function: regulation of degranulation by serine/threonine phosphatases. Pharmacol Ther 2006; 112:425–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials