Inhibition of miR301 enhances Akt-mediated cell proliferation by accumulation of PTEN in nucleus and its effects on cell-cycle regulatory proteins
- PMID: 26967567
- PMCID: PMC4991504
- DOI: 10.18632/oncotarget.7996
Inhibition of miR301 enhances Akt-mediated cell proliferation by accumulation of PTEN in nucleus and its effects on cell-cycle regulatory proteins
Abstract
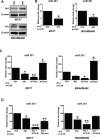
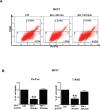
Micro-RNAs (miRs) represent an innovative class of genes that act as regulators of gene expression. Recently, the aberrant expression of several miRs has been associated with different types of cancers. In this study, we show that miR301 inhibition influences PI3K-Akt pathway activity. Akt overexpression in MCF7 and MDAMB468 cells caused downregulation of miR301 expression. This effect was confirmed by co-transfection of miR301-modulators in the presence of Akt. Cells overexpressing miR301-inhibitor and Akt, exhibited increased migration and proliferation. Experimental results also confirmed PI3K, PTEN and FoxF2 as regulatory targets for miR301. Furthermore, Akt expression in conjunction with miR301-inhibitor increased nuclear accumulation of PTEN, thus preventing it from downregulating the PI3K-signalling. In summary, our data emphasize the importance of miR301 inhibition on PI3K-Akt pathway-mediated cellular functions. Hence, it opens new avenues for the development of new anti-cancer agents preferentially targeting PI3K-Akt pathway.
Keywords: Akt; PI3K; PTEN; mTOR; miR301.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
MicroRNA-106b promotes pituitary tumor cell proliferation and invasion through PI3K/AKT signaling pathway by targeting PTEN.Tumour Biol. 2016 Oct;37(10):13469-13477. doi: 10.1007/s13277-016-5155-2. Epub 2016 Jul 27. Tumour Biol. 2016. PMID: 27465551
-
MicroRNA-214 acts as a potential oncogene in breast cancer by targeting the PTEN-PI3K/Akt signaling pathway.Int J Mol Med. 2016 May;37(5):1421-8. doi: 10.3892/ijmm.2016.2518. Epub 2016 Mar 7. Int J Mol Med. 2016. PMID: 26951965
-
Thymosin alpha 1 suppresses proliferation and induces apoptosis in breast cancer cells through PTEN-mediated inhibition of PI3K/Akt/mTOR signaling pathway.Apoptosis. 2015 Aug;20(8):1109-21. doi: 10.1007/s10495-015-1138-9. Apoptosis. 2015. PMID: 26002438
-
Nuclear phosphatidylinositol 3,4,5-trisphosphate, phosphatidylinositol 3-kinase, Akt, and PTen: emerging key regulators of anti-apoptotic signaling and carcinogenesis.Eur J Histochem. 2007;51 Suppl 1:125-31. Eur J Histochem. 2007. PMID: 17703603 Review.
-
PTEN/PTENP1: 'Regulating the regulator of RTK-dependent PI3K/Akt signalling', new targets for cancer therapy.Mol Cancer. 2018 Feb 19;17(1):37. doi: 10.1186/s12943-018-0803-3. Mol Cancer. 2018. PMID: 29455665 Free PMC article. Review.
Cited by
-
Human induced pluripotent stem cell differentiation and direct transdifferentiation into corneal epithelial-like cells.Oncotarget. 2016 Jul 5;7(27):42314-42329. doi: 10.18632/oncotarget.9791. Oncotarget. 2016. PMID: 27275539 Free PMC article.
-
Decreased expression of FOXF2 as new predictor of poor prognosis in stage I non-small cell lung cancer.Oncotarget. 2016 Aug 23;7(34):55601-55610. doi: 10.18632/oncotarget.10876. Oncotarget. 2016. PMID: 27487137 Free PMC article.
-
Role of the Forkhead box family protein FOXF2 in the progression of solid tumor: systematic review.J Cancer Res Clin Oncol. 2024 Dec 26;151(1):14. doi: 10.1007/s00432-024-06047-z. J Cancer Res Clin Oncol. 2024. PMID: 39724282 Free PMC article.
-
Structure and properties of slow-resorbing nanofibers obtained by (co-axial) electrospinning as tissue scaffolds in regenerative medicine.PeerJ. 2017 Dec 18;5:e4125. doi: 10.7717/peerj.4125. eCollection 2017. PeerJ. 2017. PMID: 29302386 Free PMC article.
-
Impact of Antibiotics on the Proliferation and Differentiation of Human Adipose-Derived Mesenchymal Stem Cells.Int J Mol Sci. 2017 Nov 24;18(12):2522. doi: 10.3390/ijms18122522. Int J Mol Sci. 2017. PMID: 29186789 Free PMC article.
References
-
- Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends in molecular medicine. 2014;20:460–469. - PubMed
-
- Mattiske S, Suetani RJ, Neilsen PM, Callen DF. The oncogenic role of miR-155 in breast cancer. Cancer epidemiology, biomarkers & prevention. 2012;21:1236–1243. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

