Generation of Antigen Microarrays to Screen for Autoantibodies in Heart Failure and Heart Transplantation
- PMID: 26967734
- PMCID: PMC4788148
- DOI: 10.1371/journal.pone.0151224
Generation of Antigen Microarrays to Screen for Autoantibodies in Heart Failure and Heart Transplantation
Abstract
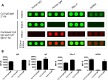
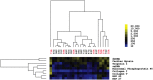
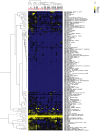
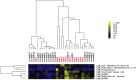
Autoantibodies directed against endogenous proteins including contractile proteins and endothelial antigens are frequently detected in patients with heart failure and after heart transplantation. There is evidence that these autoantibodies contribute to cardiac dysfunction and correlate with clinical outcomes. Currently, autoantibodies are detected in patient sera using individual ELISA assays (one for each antigen). Thus, screening for many individual autoantibodies is laborious and consumes a large amount of patient sample. To better capture the broad-scale antibody reactivities that occur in heart failure and post-transplant, we developed a custom antigen microarray technique that can simultaneously measure IgM and IgG reactivities against 64 unique antigens using just five microliters of patient serum. We first demonstrated that our antigen microarray technique displayed enhanced sensitivity to detect autoantibodies compared to the traditional ELISA method. We then piloted this technique using two sets of samples that were obtained at our institution. In the first retrospective study, we profiled pre-transplant sera from 24 heart failure patients who subsequently received heart transplants. We identified 8 antibody reactivities that were higher in patients who developed cellular rejection (2 or more episodes of grade 2R rejection in first year after transplant as defined by revised criteria from the International Society for Heart and Lung Transplantation) compared with those who did have not have rejection episodes. In a second retrospective study with 31 patients, we identified 7 IgM reactivities that were higher in heart transplant recipients who developed antibody-mediated rejection (AMR) compared with control recipients, and in time course studies, these reactivities appeared prior to overt graft dysfunction. In conclusion, we demonstrated that the autoantibody microarray technique outperforms traditional ELISAs as it uses less patient sample, has increased sensitivity, and can detect autoantibodies in a multiplex fashion. Furthermore, our results suggest that this autoantibody array technology may help to identify patients at risk of rejection following heart transplantation and identify heart transplant recipients with AMR.
Conflict of interest statement
Figures


References
-
- Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. Journal of immunology. 1987;139(11):3630–6. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

