A cationic liposome-DNA complexes adjuvant (JVRS-100) enhances the immunogenicity and cross-protective efficacy of pre-pandemic influenza A (H5N1) vaccine in ferrets
- PMID: 26967975
- PMCID: PMC5796654
- DOI: 10.1016/j.virol.2016.02.024
A cationic liposome-DNA complexes adjuvant (JVRS-100) enhances the immunogenicity and cross-protective efficacy of pre-pandemic influenza A (H5N1) vaccine in ferrets
Abstract
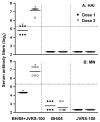
Influenza A (H5N1) viruses continue to pose a public health threat. As inactivated H5N1 vaccines are poorly immunogenic, adjuvants are needed to improve the immunogenicity of H5N1 vaccine in humans. Here, we investigated the immunogenicity and cross-protective efficacy in ferrets of a clade 2.2-derived vaccine with addition of JVRS-100, an adjuvant consisting of cationic liposome-DNA complexes (CLDC). After the first vaccination, significantly higher levels of hemagglutination-inhibition (HAI) and neutralizing antibody titers were detected in ferrets immunized with adjuvanted vaccine compared to unadjuvanted vaccine. Following a second dose of adjuvanted vaccine, HAI antibody titers of ≥ 40 were detected against viruses from multiple H5N1 clades. HAI antibodies against newly isolated H5N2 and H5N8 viruses were also augmented by JVRS-100. Ferrets were challenged with a heterologous H5N1 virus. All ferrets that received two doses of adjuvanted vaccine exhibited mild illness, significantly reduced nasal wash virus titers and protection from lethal challenge. In contrast, ferrets that received unadjuvanted vaccine showed greater weight loss, high viral titers and 3 of 6 animals succumbed to the lethal challenge. Our results indicate that the addition of JVRS-100 to H5N1 vaccine enhanced immunogenicity and cross-protection against lethal H5N1 virus disease in ferrets. JVRS-100 warrants further investigation as a potential adjuvant for influenza vaccines.
Keywords: Antibody response; CLDC adjuvant; Cross-protection; Ferrets; Influenza A (H5N1) vaccine.
Published by Elsevier Inc.
Conflict of interest statement
J.M. Katz received funding from Juvaris Inc. (Grant No. 1U01AI074512-1) to cover the cost of this research. All other authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Cationic liposome-DNA complexes (CLDC) adjuvant enhances the immunogenicity and cross-protective efficacy of a pre-pandemic influenza A H5N1 vaccine in mice.Vaccine. 2012 Jan 5;30(2):254-64. doi: 10.1016/j.vaccine.2011.10.103. Epub 2011 Nov 12. Vaccine. 2012. PMID: 22085545
-
A nanoemulsion-adjuvanted intranasal H5N1 influenza vaccine protects ferrets against homologous and heterologous H5N1 lethal challenge.Vaccine. 2019 Sep 30;37(42):6162-6170. doi: 10.1016/j.vaccine.2019.08.071. Epub 2019 Sep 5. Vaccine. 2019. PMID: 31495593
-
Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets.Vaccine. 2011 Aug 26;29(37):6242-51. doi: 10.1016/j.vaccine.2011.06.078. Epub 2011 Jul 6. Vaccine. 2011. PMID: 21736913 Free PMC article.
-
A systematic review and meta-analysis of cross-reactivity of antibodies induced by oil-in-water emulsion adjuvanted influenza H5N1 virus monovalent vaccines.Vaccine. 2017 May 31;35(24):3162-3170. doi: 10.1016/j.vaccine.2017.04.029. Epub 2017 May 5. Vaccine. 2017. PMID: 28483200
-
H5N1 vaccines in humans.Virus Res. 2013 Dec 5;178(1):78-98. doi: 10.1016/j.virusres.2013.05.006. Epub 2013 May 28. Virus Res. 2013. PMID: 23726847 Free PMC article. Review.
Cited by
-
Production and Immunogenicity of a Tag-Free Recombinant Chimera Based on PfMSP-1 and PfMSP-3 Using Alhydrogel and Dipeptide-Based Hydrogels.Vaccines (Basel). 2021 Jul 13;9(7):782. doi: 10.3390/vaccines9070782. Vaccines (Basel). 2021. PMID: 34358198 Free PMC article.
-
Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro.Int J Nanomedicine. 2017 Jul 4;12:4747-4762. doi: 10.2147/IJN.S137222. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28740382 Free PMC article.
-
A single immunization with H5N1 virus-like particle vaccine protects chickens against divergent H5N1 influenza viruses and vaccine efficacy is determined by adjuvant and dosage.Emerg Microbes Infect. 2024 Dec;13(1):2287682. doi: 10.1080/22221751.2023.2287682. Epub 2023 Dec 30. Emerg Microbes Infect. 2024. PMID: 37994795 Free PMC article.
-
Avian Influenza A Virus Pandemic Preparedness and Vaccine Development.Vaccines (Basel). 2018 Jul 25;6(3):46. doi: 10.3390/vaccines6030046. Vaccines (Basel). 2018. PMID: 30044370 Free PMC article. Review.
-
Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses.Exp Gerontol. 2019 Sep;124:110632. doi: 10.1016/j.exger.2019.110632. Epub 2019 Jun 13. Exp Gerontol. 2019. PMID: 31201918 Free PMC article.
References
-
- Bart SA, Hohenboken M, Della Cioppa G, Narasimhan V, Dormitzer PR, Kanesa-Thasan N. A cell culture-derived MF59-adjuvanted pandemic A/H7N9 vaccine is immunogenic in adults. Sci Transl Med. 2014;6:234ra255. - PubMed
-
- Belshe RB, Frey SE, Graham IL, Anderson EL, Jackson LA, Spearman P, Edupuganti S, Mulligan MJ, Rouphael N, Winokur P, Dolor RJ, Woods CW, Walter EB, Chen WH, Turley C, Edwards KM, Creech CB, Hill H, Bellamy AR National Institute of Allergy and Infectious Diseases–Funded Vaccine and Treatment Evaluation Units. Immunogenicity of avian influenza A/Anhui/01/2005(H5N1) vaccine with MF59 adjuvant: a randomized clinical trial. JAMA: J Am Med Assoc. 2014;312:1420–1428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

