Epigallocatechin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation
- PMID: 26968114
- PMCID: PMC5848242
- DOI: 10.1016/j.ajpath.2015.12.004
Epigallocatechin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation
Abstract
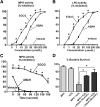
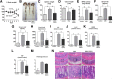
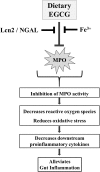
Green tea-derived polyphenol (-)-epigallocatechin-3-gallate (EGCG) has been extensively studied for its antioxidant and anti-inflammatory properties in models of inflammatory bowel disease, yet the underlying molecular mechanism is not completely understood. Herein, we demonstrate that EGCG can potently inhibit the proinflammatory enzyme myeloperoxidase in vitro in a dose-dependent manner over a range of physiologic temperatures and pH values. The ability of EGCG to mediate its inhibitory activity is counter-regulated by the presence of iron and lipocalin 2. Spectral analysis indicated that EGCG prevents the peroxidase-catalyzed reaction by reverting the reactive peroxidase heme (compound I:oxoiron) back to its native inactive ferric state, possibly via the exchange of electrons. Further, administration of EGCG to dextran sodium sulfate-induced colitic mice significantly reduced the colonic myeloperoxidase activity and alleviated proinflammatory mediators associated with gut inflammation. However, the efficacy of EGCG against gut inflammation is diminished when orally coadministered with iron. These findings indicate that the ability of EGCG to inhibit myeloperoxidase activity is one of the mechanisms by which it exerts mucoprotective effects and that counter-regulatory factors such as dietary iron and luminal lipocalin 2 should be taken into consideration for optimizing clinical management strategies for inflammatory bowel disease with the use of EGCG treatment.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut.Nat Commun. 2015 May 12;6:7113. doi: 10.1038/ncomms8113. Nat Commun. 2015. PMID: 25964185 Free PMC article.
-
Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis.J Crohns Colitis. 2012 Mar;6(2):226-35. doi: 10.1016/j.crohns.2011.08.012. Epub 2011 Sep 23. J Crohns Colitis. 2012. PMID: 22325177
-
EGCG inhibit chemical reactivity of iron through forming an Ngal-EGCG-iron complex.Biometals. 2013 Dec;26(6):1041-50. doi: 10.1007/s10534-013-9681-8. Epub 2013 Oct 26. Biometals. 2013. PMID: 24158698 Free PMC article.
-
The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer.Biochim Biophys Acta. 2012 Aug;1826(1):129-69. doi: 10.1016/j.bbcan.2012.03.008. Epub 2012 Mar 31. Biochim Biophys Acta. 2012. PMID: 22513004 Free PMC article. Review.
-
Therapeutic effects of epigallocatechin-3-gallate for inflammatory bowel disease: A preclinical meta-analysis.Phytomedicine. 2024 Jun;128:155408. doi: 10.1016/j.phymed.2024.155408. Epub 2024 Feb 2. Phytomedicine. 2024. PMID: 38503153
Cited by
-
Gut Microbiota Conversion of Dietary Ellagic Acid into Bioactive Phytoceutical Urolithin A Inhibits Heme Peroxidases.PLoS One. 2016 Jun 2;11(6):e0156811. doi: 10.1371/journal.pone.0156811. eCollection 2016. PLoS One. 2016. PMID: 27254317 Free PMC article.
-
Low dose Epigallocatechin Gallate Alleviates Experimental Colitis by Subduing Inflammatory Cells and Cytokines, and Improving Intestinal Permeability.Nutrients. 2019 Jul 29;11(8):1743. doi: 10.3390/nu11081743. Nutrients. 2019. PMID: 31362373 Free PMC article.
-
Catechin Protects against Lipopolysaccharide-induced Depressive-like Behaviour in Mice by Regulating Neuronal and Inflammatory Genes.Curr Gene Ther. 2024;24(4):292-306. doi: 10.2174/0115665232261045231215054305. Curr Gene Ther. 2024. PMID: 38783529
-
Lipocalin-2 and intestinal diseases.World J Gastroenterol. 2024 Dec 14;30(46):4864-4879. doi: 10.3748/wjg.v30.i46.4864. World J Gastroenterol. 2024. PMID: 39679305 Free PMC article. Review.
-
Determination of the Inhibitory Potential of Chalcones on Myeloperoxidase Enzyme Activity: In vitro and Molecular Docking Studies.Cell Biochem Biophys. 2025 Mar 13. doi: 10.1007/s12013-025-01719-0. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40080351
References
-
- Dragicevic N., Smith A., Lin X., Yuan F., Copes N., Delic V., Tan J., Cao C., Shytle R.D., Bradshaw P.C. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer's amyloid-induced mitochondrial dysfunction. J Alzheimers Dis. 2011;26:507–521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

