Most microRNAs in the single-cell alga Chlamydomonas reinhardtii are produced by Dicer-like 3-mediated cleavage of introns and untranslated regions of coding RNAs
- PMID: 26968199
- PMCID: PMC4817775
- DOI: 10.1101/gr.199703.115
Most microRNAs in the single-cell alga Chlamydomonas reinhardtii are produced by Dicer-like 3-mediated cleavage of introns and untranslated regions of coding RNAs
Abstract
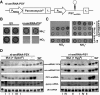
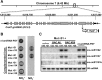
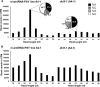
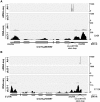
We describe here a forward genetic screen to investigate the biogenesis, mode of action, and biological function of miRNA-mediated RNA silencing in the model algal species,Chlamydomonas reinhardtii Among the mutants from this screen, there were three at Dicer-like 3 that failed to produce both miRNAs and siRNAs and others affecting diverse post-biogenesis stages of miRNA-mediated silencing. The DCL3-dependent siRNAs fell into several classes including transposon- and repeat-derived siRNAs as in higher plants. The DCL3-dependent miRNAs differ from those of higher plants, however, in that many of them are derived from mRNAs or from the introns of pre-mRNAs. Transcriptome analysis of the wild-type and dcl3 mutant strains revealed a further difference from higher plants in that the sRNAs are rarely negative switches of mRNA accumulation. The few transcripts that were more abundant in dcl3 mutant strains than in wild-type cells were not due to sRNA-targeted RNA degradation but to direct DCL3 cleavage of miRNA and siRNA precursor structures embedded in the untranslated (and translated) regions of the mRNAs. Our analysis reveals that the miRNA-mediated RNA silencing in C. reinhardtii differs from that of higher plants and informs about the evolution and function of this pathway in eukaryotes.
© 2016 Valli et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


Similar articles
-
Physcomitrella patens DCL3 is required for 22-24 nt siRNA accumulation, suppression of retrotransposon-derived transcripts, and normal development.PLoS Genet. 2008 Dec;4(12):e1000314. doi: 10.1371/journal.pgen.1000314. Epub 2008 Dec 19. PLoS Genet. 2008. PMID: 19096705 Free PMC article.
-
RNA-binding protein DUS16 plays an essential role in primary miRNA processing in the unicellular alga Chlamydomonas reinhardtii.Proc Natl Acad Sci U S A. 2016 Sep 20;113(38):10720-5. doi: 10.1073/pnas.1523230113. Epub 2016 Aug 31. Proc Natl Acad Sci U S A. 2016. PMID: 27582463 Free PMC article.
-
Complementarity to an miRNA seed region is sufficient to induce moderate repression of a target transcript in the unicellular green alga Chlamydomonas reinhardtii.Plant J. 2013 Dec;76(6):1045-56. doi: 10.1111/tpj.12354. Plant J. 2013. PMID: 24127635
-
Intron-mediated RNA interference and microRNA biogenesis.Methods Mol Biol. 2009;487:387-413. doi: 10.1007/978-1-60327-547-7_19. Methods Mol Biol. 2009. PMID: 19301658 Review.
-
Mechanisms of microRNA-mediated gene regulation in unicellular model alga Chlamydomonas reinhardtii.Biotechnol Biofuels. 2018 Sep 8;11:244. doi: 10.1186/s13068-018-1249-y. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 30202439 Free PMC article. Review.
Cited by
-
Genetic Insight into the Domain Structure and Functions of Dicer-Type Ribonucleases.Int J Mol Sci. 2021 Jan 9;22(2):616. doi: 10.3390/ijms22020616. Int J Mol Sci. 2021. PMID: 33435485 Free PMC article. Review.
-
The evolutionary origin of plant and animal microRNAs.Nat Ecol Evol. 2017 Feb 21;1(3):27. doi: 10.1038/s41559-016-0027. Nat Ecol Evol. 2017. PMID: 28529980 Free PMC article.
-
Cooperative processing of primary miRNAs by DUS16 and DCL3 in the unicellular green alga Chlamydomonas reinhardtii.Commun Integr Biol. 2017 Feb 6;10(1):e1280208. doi: 10.1080/19420889.2017.1280208. eCollection 2017. Commun Integr Biol. 2017. PMID: 28289490 Free PMC article.
-
Global identification of AGO3-RNA interactions reveals targets of small RNA-mediated gene regulation in Chlamydomonas reinhardtii.Plant Cell Physiol. 2025 Jul 24;66(6):940-955. doi: 10.1093/pcp/pcaf040. Plant Cell Physiol. 2025. PMID: 40244707 Free PMC article.
-
Biogenesis, conservation, and function of miRNA in liverworts.J Exp Bot. 2022 Jul 16;73(13):4528-4545. doi: 10.1093/jxb/erac098. J Exp Bot. 2022. PMID: 35275209 Free PMC article. Review.
References
-
- Baulcombe D. 2004. RNA silencing in plants. Nature 431: 356–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
