C/EBPβ regulates sensitivity to bortezomib in prostate cancer cells by inducing REDD1 and autophagosome-lysosome fusion
- PMID: 26968249
- PMCID: PMC4818955
- DOI: 10.1016/j.canlet.2016.03.005
C/EBPβ regulates sensitivity to bortezomib in prostate cancer cells by inducing REDD1 and autophagosome-lysosome fusion
Abstract
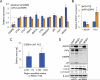
The purpose of this study was to ascertain the mechanisms by which advanced prostate cancer cells resist bortezomib therapy. Several independent studies have shown that cells are protected from proteasome inhibition by increased autophagic activity. We investigated whether C/EBPβ, a transcription factor involved in the control of autophagic gene expression, regulates resistance to proteasome inhibition. In PC3 cells over-expressing C/EBPβ, turnover of autophagic substrates and expression of core autophagy genes were increased. Conversely, C/EBPβ knockdown suppressed autophagosome-lysosome fusion. We also found that C/EBPβ knockdown suppressed REDD1 expression to delay early autophagy, an effect rescued by exogenous REDD1. Cells with suppressed C/EBPβ levels showed delayed autophagy activation upon bortezomib treatment. Knockdown of C/EBPβ sensitized PC3 cells to bortezomib, and blockade of autophagy by chloroquine did not further increase cell death in cells expressing shRNA targeting C/EBPβ. Lastly, we observed a decreased growth of PC3 cells and xenografts with C/EBPβ knockdown and such xenografts were sensitized to bortezomib treatment. Our results demonstrate that C/EBPβ is a critical effector of autophagy via regulation of autolysosome formation and promotes resistance to proteasome inhibitor treatment by increasing autophagy.
Keywords: Autophagy; C/EBPβ; Prostate cancer; Proteasome inhibitor; REDD1.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Figures





References
-
- Morris MJ, Kelly WK, Slovin S, Ryan C, Eicher C, Heller G, et al. A phase II trial of bortezomib and prednisone for castration resistant metastatic prostate cancer. J. Urol. 2007;178:2378–2383. - PubMed
-
- Dreicer R, Petrylak D, Agus D, Webb I, Roth B. Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin. Cancer Res. 2007;13:1208–1215. - PubMed
-
- Hainsworth JD, Meluch AA, Spigel DR, Barton J, Jr., Simons L, Meng C, et al. Weekly docetaxel and bortezomib as first-line treatment for patients with hormone-refractory prostate cancer: a Minnie Pearl Cancer Research Network phase II trial. Clin. Genitourin. Cancer. 2007;5:278–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

