Bioenergetics and redox adaptations of astrocytes to neuronal activity
- PMID: 26968531
- PMCID: PMC5018236
- DOI: 10.1111/jnc.13486
Bioenergetics and redox adaptations of astrocytes to neuronal activity
Abstract
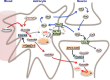
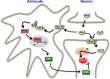
Neuronal activity is a high-energy demanding process recruiting all neural cells that adapt their metabolism to sustain the energy and redox balance of neurons. During neurotransmission, synaptic cleft glutamate activates its receptors in neurons and in astrocytes, before being taken up by astrocytes through energy costly transporters. In astrocytes, the energy requirement for glutamate influx is likely to be met by glycolysis. To enable this, astrocytes are constitutively glycolytic, robustly expressing 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3), an enzyme that is negligibly present in neurons by continuous degradation because of the ubiquitin-proteasome pathway via anaphase-promoting complex/cyclosome (APC)-Cdh1. Additional factors contributing to the glycolytic frame of astrocytes may include 5'-AMP-activated protein kinase (AMPK), hypoxia-inducible factor-1 (HIF-1), pyruvate kinase muscle isoform-2 (PKM2), pyruvate dehydrogenase kinase-4 (PDK4), lactate dehydrogenase-B, or monocarboxylate transporter-4 (MCT4). Neurotransmission-associated messengers, such as nitric oxide or ammonium, stimulate lactate release from astrocytes. Astrocyte-derived glycolytic lactate thus sustains the energy needs of neurons, which in contrast to astrocytes mainly rely on oxidative phosphorylation. Neuronal activity unavoidably triggers reactive oxygen species, but the antioxidant defense of neurons is weak; hence, they use glucose for oxidation through the pentose-phosphate pathway to preserve the redox status. Furthermore, neural activity is coupled with erythroid-derived erythroid-derived 2-like 2 (Nrf2) mediated transcriptional activation of antioxidant genes in astrocytes, which boost the de novo glutathione biosynthesis in neighbor neurons. Thus, the bioenergetics and redox programs of astrocytes are adapted to sustain neuronal activity and survival. Developing therapeutic strategies to interfere with these pathways may be useful to combat neurological diseases. Our current knowledge on brain's management of bioenergetics and redox requirements associated with neural activity is herein revisited. The astrocyte-neuronal lactate shuttle (ANLS) explains the energy needs of neurotransmission. Furthermore, neurotransmission unavoidably triggers increased mitochondrial reactive oxygen species in neurons. By coupling glutamatergic activity with transcriptional activation of antioxidant genes, astrocytes provide neurons with neuroprotective glutathione through an astrocyte-neuronal glutathione shuttle (ANGS). This article is part of the 60th Anniversary special issue.
Keywords: AMPK; GSH; Cdh1; Glycolysis; Nrf2; PFKFB3.
© 2016 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures
References
-
- Allaman I., Belanger M. and Magistretti P. J. (2011) Astrocyte‐neuron metabolic relationships: for better and for worse. Trends Neurosci. 34, 76–87. - PubMed
-
- Allen N. J. and Barres B. A. (2009) Neuroscience: Glia ‐ more than just brain glue. Nature 457, 675–677. - PubMed
-
- Almeida A. and Bolaños J. P. (2001) A transient inhibition of mitochondrial ATP synthesis by nitric oxide synthase activation triggered apoptosis in primary cortical neurons. J. Neurochem. 77, 676–690. - PubMed
-
- Almeida A., Moncada S. and Bolaños J. P. (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6‐phosphofructo‐2‐kinase pathway. Nat. Cell Biol. 6, 45–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

