Povidone-iodine ointment demonstrates in vitro efficacy against biofilm formation
- PMID: 26968574
- PMCID: PMC7949843
- DOI: 10.1111/iwj.12578
Povidone-iodine ointment demonstrates in vitro efficacy against biofilm formation
Abstract
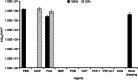
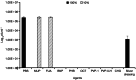
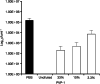
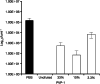
Anti-infectives used to treat chronic exuding wounds are diluted by wound exudates, absorbed into dressings, metabolised by proteases and destroyed by pH. In order to mimic such effects of exudates, the efficacy of six topical wound agents was assessed undiluted and at 10% concentrations, including povidone-iodine ointment and a silver-impregnated wound dressing, to remove biofilms of Pseudomonas aeruginosa, multi-species biofilms of Candida albicans and methicillin-resistant Staphylococcus aureus (MRSA) in vitro in a Centers for Disease Control and Prevention (CDC) reactor. Povidone-iodine was also diluted to 3·3% and 33·3% of the commercial concentrations. Viable microorganisms in each preparation were quantified by colony count. No viable P. aeruginosa biofilm material was recovered after 4 and 24 hours of treatment with povidone-iodine ointment at the 100% and 10% concentrations. No C. albicans/MRSA biofilm material was recovered after 4 and 24 hours of treatment with povidone-iodine ointment at the 100% concentration. In general, following dilution, povidone-iodine ointment appeared to exhibit greater biofilm removal than the other agents tested. Further research involving different microorganisms in vitro and in vivo over a longer period of time will help elucidate the full potential of povidone-iodine ointment and liposomal hydrogel.
Keywords: Biofilm; Candida albicans; Methicillin-resistant Staphylococcus aureus (MRSA); Povidone-iodine ointment (PVP-I); Pseudomonas aeruginosa.
© 2016 The Authors. International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Figures


Similar articles
-
Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00682-20. doi: 10.1128/AAC.00682-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32571829 Free PMC article. Review.
-
Efficacy of common antiseptic solutions against clinically relevant microorganisms in biofilm.Bone Joint J. 2021 May;103-B(5):908-915. doi: 10.1302/0301-620X.103B5.BJJ-2020-1245.R2. Bone Joint J. 2021. PMID: 33934664
-
Modelling antisepsis using defined populations of facultative and anaerobic wound pathogens grown in a basally perfused biofilm model.Biofouling. 2018 May;34(5):507-518. doi: 10.1080/08927014.2018.1466115. Epub 2018 Jun 6. Biofouling. 2018. PMID: 29873244
-
Povidone-iodine and silver nitrate are equally effective in eradicating staphylococcal biofilm grown on a titanium surface: an in-vitro analysis.J Hosp Infect. 2025 Jan;155:185-191. doi: 10.1016/j.jhin.2024.11.012. Epub 2024 Nov 22. J Hosp Infect. 2025. PMID: 39579939
-
Role of antiseptics in the prevention and treatment of infections in nursing homes.J Hosp Infect. 2023 Jan;131:58-69. doi: 10.1016/j.jhin.2022.09.021. Epub 2022 Oct 8. J Hosp Infect. 2023. PMID: 36216172 Review.
Cited by
-
A Three-Dimensional Model of Bacterial Biofilms and Its Use in Antimicrobial Susceptibility Testing.Microorganisms. 2024 Jan 19;12(1):203. doi: 10.3390/microorganisms12010203. Microorganisms. 2024. PMID: 38276189 Free PMC article.
-
Topical Antibiofilm Agents With Potential Utility in the Treatment of Chronic Rhinosinusitis: A Narrative Review.Front Pharmacol. 2022 Jun 13;13:840323. doi: 10.3389/fphar.2022.840323. eCollection 2022. Front Pharmacol. 2022. PMID: 35770097 Free PMC article. Review.
-
Molecular iodine exerts antineoplastic effects by diminishing proliferation and invasive potential and activating the immune response in mammary cancer xenografts.BMC Cancer. 2019 Mar 22;19(1):261. doi: 10.1186/s12885-019-5437-3. BMC Cancer. 2019. PMID: 30902074 Free PMC article.
-
Evaluation of short exposure times of antimicrobial wound solutions against microbial biofilms: from in vitro to in vivo.J Antimicrob Chemother. 2018 Feb 1;73(2):494-502. doi: 10.1093/jac/dkx391. J Antimicrob Chemother. 2018. PMID: 29165561 Free PMC article.
-
Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00682-20. doi: 10.1128/AAC.00682-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32571829 Free PMC article. Review.
References
-
- Crovetti G, Martinelli G, Issi M, Barone M, Guizzard M, Campanati B, Moroni M, Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci 2004;30:145–51. - PubMed
-
- Falanga V. The chronic wound: impaired healing and solutions in the context of wound bed preparation. Blood Cells Mol Dis 2004;32:88–94. - PubMed
-
- Bryant RA, Nix DP. Acute and chronic wounds: current management concepts, 4th edn. Mosby, Elsevier, 2011.
-
- Dow G, Browne A, Sibbald RG. Infection in chronic wounds: controversies in diagnosis and treatment. Ostomy Wound Manage 1999;45:23–7, 29–40; quiz 41–2. - PubMed
-
- Kingsley A. The wound infection continuum and its application to clinical practice. Ostomy Wound Manage 2003;49(Suppl. 7A):1–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

