Mometasone furoate in the treatment of mild, moderate, or severe persistent allergic rhinitis: a non-inferiority study (PUMA)
- PMID: 26968623
- PMCID: PMC9444672
- DOI: 10.1016/j.bjorl.2015.11.009
Mometasone furoate in the treatment of mild, moderate, or severe persistent allergic rhinitis: a non-inferiority study (PUMA)
Abstract
Introduction: Allergic rhinitis is considered the most prevalent respiratory disease in Brazil and worldwide, with great impact on quality of life, affecting social life, sleep, and also performance at school and at work.
Objective: To compare the efficacy and safety of two formulations containing mometasone furoate in the treatment of mild, moderate, or severe persistent allergic rhinitis after four weeks of treatment.
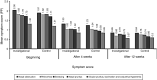
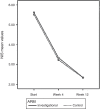
Methods: Phase III, randomized, non-inferiority, national, open study comparing mometasone furoate in two presentations (control drug and investigational drug). The primary endpoint was the percentage of patients with reduction of at least 0.55 in nasal index score (NIS) after four weeks of treatment. Secondary outcomes included total nasal index score score after four and 12 weeks of treatment; individual scores for symptoms of nasal obstruction, rhinorrhea, sneezing, and nasal pruritus; as well as score for pruritus, lacrimation, and ocular redness after four and 12 weeks of treatment. The study was registered at clinicaltrials.gov with the reference number NCT01372865.
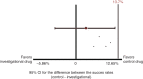
Results: The efficacy primary analysis demonstrated non-inferiority of the investigational drug in relation to the control drug, since the upper limit of the confidence interval (CI) of 95% for the difference between the success rates after four weeks of treatment (12.6%) was below the non-inferiority margin provided during the determination of the sample size (13.7%). Adverse events were infrequent and with mild intensity in most cases.
Conclusion: The efficacy and safety of investigational drug in the treatment of persistent allergic rhinitis were similar to the reference product, demonstrating its non-inferiority.
Introdução: A rinite alérgica é considerada a doença respiratória mais prevalente no Brasil e em todo o mundo, com grande impacto na qualidade de vida; além de, afetar a vida social, o sono e também o desempenho na escola e no trabalho.
Objetivo: Comparar a eficácia e segurança de duas formulações contendo furoato de mometasona no tratamento da rinite alérgica persistente leve, moderada ou grave por um período de quatro semanas.
Método: Trata-se de um estudo nacional aberto de fase III, randomizado, de não inferioridade de comparação do furoato de mometasona em duas apresentações (medicação de controle e fármaco sob investigação). O ponto final primário foi o percentual de pacientes com redução mínima de 0,55 no escore de índice nasal (EIN) após quatro semanas de tratamento. Os desfechos secundários foram: escore NIS total após 4 e 12 semanas de tratamento; escores individuais para sintomas de obstrução nasal, rinorréia, espirros e prurido nasal, bem como escores para prurido, lacrimejamento e hiperemia conjuntival após 4 e 12 semanas de tratamento. O estudo foi registrado em clinicaltrials.gov com o número de referência NCT01372865.
Resultados: A análise de eficácia primária demonstrou não inferioridade do fármaco sob investigação em relação à medicação de controle, visto que o limite superior do intervalo de confiança (IC) de 95% para a diferença entre os percentuais de sucesso após quatro semanas de tratamento (12,6%) situava-se abaixo da margem de não inferioridade proporcionada durante a determinação do tamanho da amostra (13,7%). Eventos adversos foram pouco frequentes e de leve intensidade na maioria dos casos.
Conclusão: A eficácia e a segurança de um fármaco experimental no tratamento da rinite alérgica persistente foram similares às do produto de referência, o que demonstrou sua não inferioridade.
Keywords: Furoato de mometasona; Mometasone furoate; Non-inferiority; Não inferioridade; Persistent allergic rhinitis; Rinite alérgica persistente.
Copyright © 2016 Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial. Published by Elsevier Editora Ltda. All rights reserved.
Figures
References
-
- Bousquet J., Khaltaev N., Cruz A.A., Denburg J., Fokkens W.J., Toqias A., et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update. Allergy. 2008;(Suppl. 86):8–160. - PubMed
-
- Plaut M., Valentine M.D. Clinical practice: allergic rhinitis. N Engl J Med. 2005;353:1934–1944. - PubMed
-
- Ibiapina Cda.C., Sarinho E.S., Camargos P.A., Andrade C.R., Cruz Filho A.A. Allergic rhinitis: epidemiological aspects, diagnosis and treatment. J Bras Pneumol. 2008;34:230–240. - PubMed
-
- Dykewicz M.S., Fineman S. Executive summary of joint task force practice parameters on diagnosis and management of rhinitis. Ann Allergy Asthma Immunol. 1998;81:463–468. - PubMed
-
- Lima R.G., Pastorino A.C., Casagrande R.R., Sole D., Leone C., Jacob C.M. Prevalence of asthma, rhinitis and eczema in 6–7 years old students from the western districts of São Paulo city, using the standardized questionnaire of the “International Study of Asthma and Allergies in Childhood” (ISAAC)-phase IIIB. Clinics. 2007;62:225–234. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

