Multi-objective optimization framework to obtain model-based guidelines for tuning biological synthetic devices: an adaptive network case
- PMID: 26968941
- PMCID: PMC4788947
- DOI: 10.1186/s12918-016-0269-0
Multi-objective optimization framework to obtain model-based guidelines for tuning biological synthetic devices: an adaptive network case
Abstract
Background: Model based design plays a fundamental role in synthetic biology. Exploiting modularity, i.e. using biological parts and interconnecting them to build new and more complex biological circuits is one of the key issues. In this context, mathematical models have been used to generate predictions of the behavior of the designed device. Designers not only want the ability to predict the circuit behavior once all its components have been determined, but also to help on the design and selection of its biological parts, i.e. to provide guidelines for the experimental implementation. This is tantamount to obtaining proper values of the model parameters, for the circuit behavior results from the interplay between model structure and parameters tuning. However, determining crisp values for parameters of the involved parts is not a realistic approach. Uncertainty is ubiquitous to biology, and the characterization of biological parts is not exempt from it. Moreover, the desired dynamical behavior for the designed circuit usually results from a trade-off among several goals to be optimized.
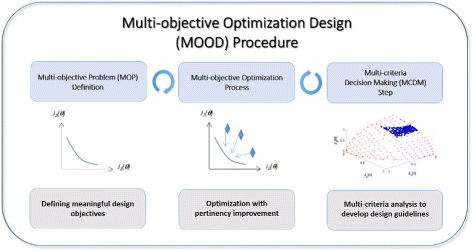
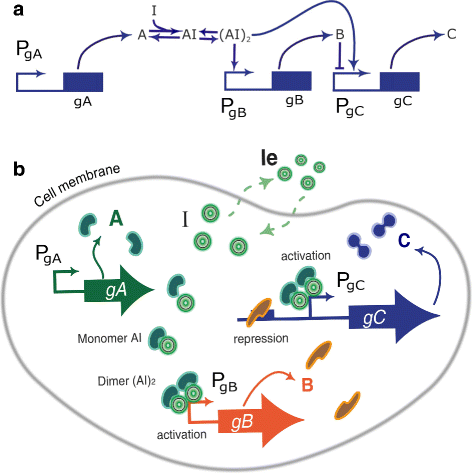
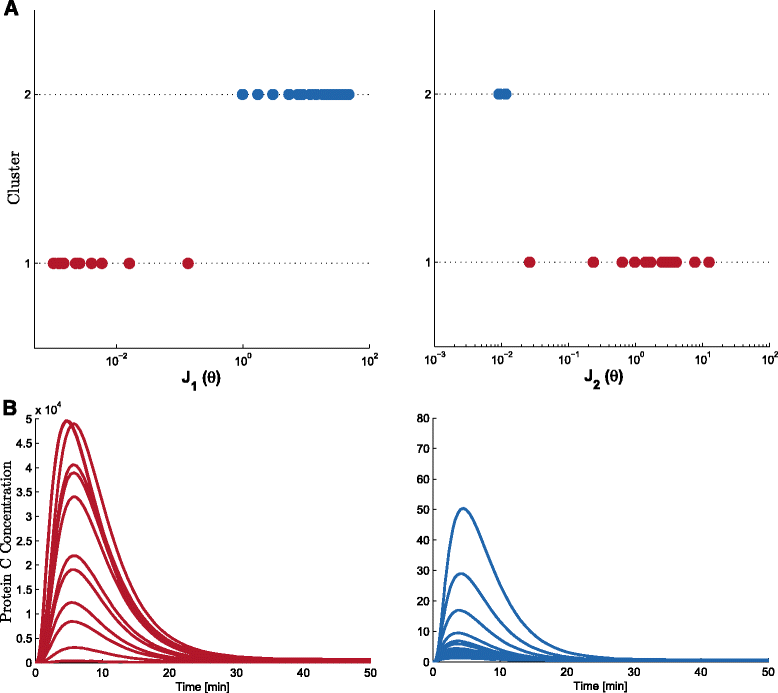
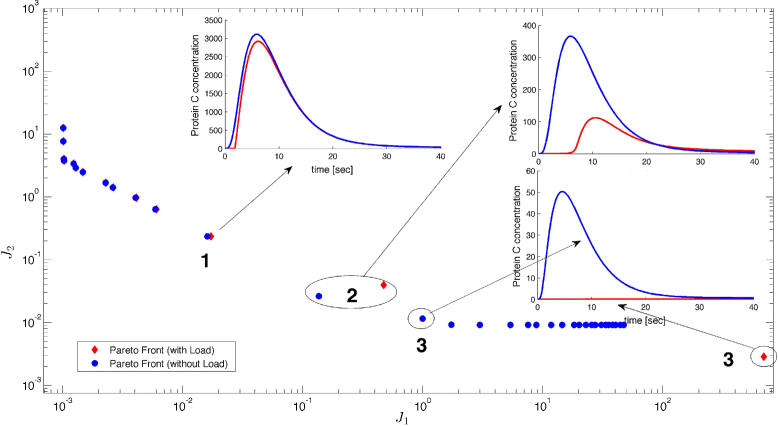
Results: We propose the use of a multi-objective optimization tuning framework to get a model-based set of guidelines for the selection of the kinetic parameters required to build a biological device with desired behavior. The design criteria are encoded in the formulation of the objectives and optimization problem itself. As a result, on the one hand the designer obtains qualitative regions/intervals of values of the circuit parameters giving rise to the predefined circuit behavior; on the other hand, he obtains useful information for its guidance in the implementation process. These parameters are chosen so that they can effectively be tuned at the wet-lab, i.e. they are effective biological tuning knobs. To show the proposed approach, the methodology is applied to the design of a well known biological circuit: a genetic incoherent feed-forward circuit showing adaptive behavior.
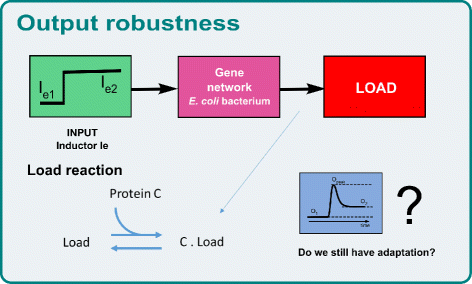
Conclusion: The proposed multi-objective optimization design framework is able to provide effective guidelines to tune biological parameters so as to achieve a desired circuit behavior. Moreover, it is easy to analyze the impact of the context on the synthetic device to be designed. That is, one can analyze how the presence of a downstream load influences the performance of the designed circuit, and take it into account.
Keywords: Biological circuits; Biological tuning knobs; Dynamic behavior; Kinetic parameters; Multi-objective optimization.
Figures




References
-
- ERASynBio. Next steps for european synthetic biology: a strategic vision from erasynbio. Report, ERASynBio. 2014. https://www.erasynbio.eu/lw_resource/datapool/_items/item_58/erasynbiost....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

