Aspirin induces Nrf2-mediated transcriptional activation of haem oxygenase-1 in protection of human melanocytes from H2 O2 -induced oxidative stress
- PMID: 26969214
- PMCID: PMC4929306
- DOI: 10.1111/jcmm.12812
Aspirin induces Nrf2-mediated transcriptional activation of haem oxygenase-1 in protection of human melanocytes from H2 O2 -induced oxidative stress
Abstract
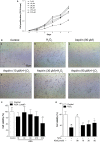
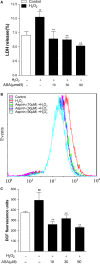
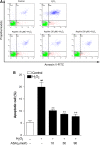
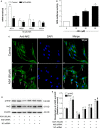
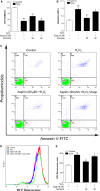
The removal of hydrogen peroxide (H2 O2 ) by antioxidants has been proven to be beneficial to patients with vitiligo. Aspirin (acetylsalicylic acid, ASA) has antioxidant activity and has great preventive and therapeutical effect in many oxidative stress-relevant diseases. Whether ASA can protect human melanocytes against oxidative stress needs to be further studied. Here, we investigated the potential protective effect and mechanisms of ASA against H2 O2 -induced oxidative injury in human melanocytes. Human melanocytes were pre-treated with different concentrations of ASA, followed by exposure to 1.0 mM H2 O2 . Cell apoptosis, intracellular reactive oxygen species (ROS) levels were evaluated by flow cytometry, and cell viability was determined by an Cell Counting Kit-8 assay. Total and phosphorylated NRF2 expression, NRF2 nuclear translocation and antioxidant response element (ARE) transcriptional activity were assayed with or without Nrf2-siRNA transfection to investigate the possible molecular mechanisms. Concomitant with an increase in viability, pre-treatment of 10-90 μmol/l ASA resulted in decreased rate of apoptotic cells, lactate dehydrogenase release and intracellular ROS levels in primary human melanocytes. Furthermore, we found ASA dramatically induced NRF2 nuclear translocation, enhanced ARE-luciferase activity, increased both p- NRF2 and total NRF2 levels, and induced the expression of haem oxygenase-1 (HO-1) in human melanocytes. In addition, knockdown of Nrf2 expression or pharmacological inhibition of HO-1 abrogated the protective action of ASA on melanocytes against H2 O2 -induced cytotoxicity and apoptosis. These results suggest that ASA protects human melanocytes against H2 O2 -induced oxidative stress via Nrf2-driven transcriptional activation of HO-1.
Keywords: aspirin; haem oxygenase-1; hydrogen peroxide; melanocyte; nuclear factor E2-related factor 2; oxidative stress; vitiligo.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Halder RM, Chappell JL. Vitiligo update. Semin Cutan Med Surg. 2009; 28: 86–92. - PubMed
-
- Schallreuter KU, Bahadoran P, Picardo M, et al Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else? Exp Dermatol. 2008; 17: 139–40, 141–60. - PubMed
-
- Picardo M. Bastonini. A new view of vitiligo: looking at normal‐appearing skin. J Invest Dermatol. 2015; 135: 1713–4. - PubMed
-
- Zhou Z, Li CY, Li K, et al Decreased methionine sulphoxide reductase A expression renders melanocytes more sensitive to oxidative stress: a possible cause for melanocyte loss in vitiligo. Br J Dermatol. 2009; 161: 504–9. - PubMed
-
- Guan CP, Wei XD, Chen HY, et al Abnormal nuclear translocation of nuclear factor‐E2 related factor 2 in the lesion of vitiligo. Zhonghua Yi Xue Za Zhi. 2008; 88: 2403–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

