Different rate-limiting activities of intracellular pH regulators for HCO3- secretion stimulated by forskolin and carbachol in rat parotid intralobular ducts
- PMID: 26969473
- PMCID: PMC10717326
- DOI: 10.1007/s12576-016-0443-6
Different rate-limiting activities of intracellular pH regulators for HCO3- secretion stimulated by forskolin and carbachol in rat parotid intralobular ducts
Abstract
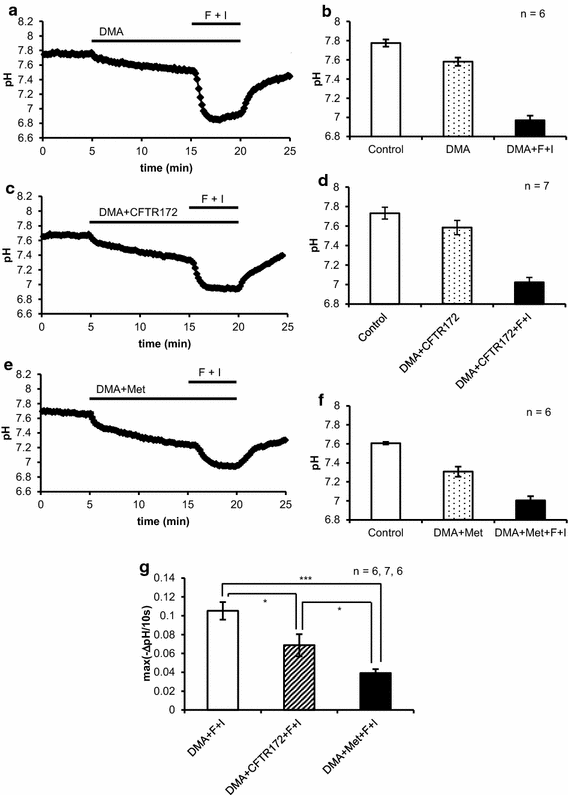
Intracellular pH (pHi) regulation fundamentally participates in maintaining HCO3- release from HCO3--secreting epithelia. We used parotid intralobular ducts loaded with BCECF to investigate the contributions of a carbonic anhydrase (CA), anion channels and a Na+-H+ exchanger (NHE) to pHi regulation for HCO3- secretion by cAMP and Ca2+ signals. Resting pHi was dispersed between 7.4 and 7.9. Forskolin consistently decreased pHi showing the dominance of pHi-lowering activities, but carbachol gathered pHi around 7.6. CA inhibition suppressed the forskolin-induced decrease in pHi, while it allowed carbachol to consistently increase pHi by revealing that carbachol prominently activated NHE via Ca2+-calmodulin. Under NHE inhibition, forskolin and carbachol induced the remarkable decreases in pHi, which were slowed predominantly by CA inhibition and by CA or anion channel inhibition, respectively. Our results suggest that forskolin and carbachol primarily activate the pHi-lowering CA and pHi-raising NHE, respectively, to regulate pHi for HCO3- secretion.
Keywords: Bicarbonate secretion; Carbonic anhydorase; Cl− channels; Intracellular pH; Na+–H+ exchanger; Rat parotid intralobular ducts.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
-
- Cook DI, Van Lennep EW, Roberts ML, Young JA. Secretion by the major salivary glands. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 3. New York: Raven Press; 1994. pp. 1061–1117.
-
- Catalán MA, Kondo Y, Peña-Munzenmayer G, Jaramillo Y, Liu F, Choi S, Crandall E, Borok Z, Flodby P, Shull GE, Melvin JE. A fluid secretion pathway unmasked by acinar-specific Tmem16A gene ablation in the adult mouse salivary gland. Proc Natl Acad Sci USA. 2015;112:2263–2268. doi: 10.1073/pnas.1415739112. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

