Durable Clinical Effectiveness With Paclitaxel-Eluting Stents in the Femoropopliteal Artery: 5-Year Results of the Zilver PTX Randomized Trial
- PMID: 26969758
- PMCID: PMC4823823
- DOI: 10.1161/CIRCULATIONAHA.115.016900
Durable Clinical Effectiveness With Paclitaxel-Eluting Stents in the Femoropopliteal Artery: 5-Year Results of the Zilver PTX Randomized Trial
Erratum in
-
Correction to: Durable Clinical Effectiveness With Paclitaxel-Eluting Stents in the Femoropopliteal Artery 5-Year Results of the Zilver PTX Randomized Trial.Circulation. 2019 Feb 19;139(8):e42. doi: 10.1161/CIR.0000000000000657. Circulation. 2019. PMID: 30779646 Free PMC article. No abstract available.
Abstract
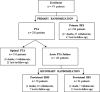
Background: This randomized controlled trial evaluated clinical durability of Zilver PTX, a paclitaxel-coated drug-eluting stent (DES), for femoropopliteal artery lesions. Outcomes compare primary DES versus percutaneous transluminal angioplasty (PTA), overall DES (primary and provisional) versus standard care (PTA and provisional Zilver bare metal stent [BMS]), and provisional DES versus provisional BMS.
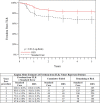
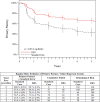
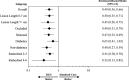
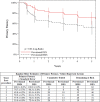
Methods and results: Patients with symptomatic femoropopliteal artery disease were randomly assigned to DES (n=236) or PTA (n=238). Approximately 91% had claudication; 9% had critical limb ischemia. Patients experiencing acute PTA failure underwent secondary randomization to provisional BMS (n=59) or DES (n=61). The 1-year primary end points of event-free survival and patency showed superiority of primary DES in comparison with PTA; these results were sustained through 5 years. Clinical benefit (freedom from persistent or worsening symptoms of ischemia; 79.8% versus 59.3%, P<0.01), patency (66.4% versus 43.4%, P<0.01), and freedom from reintervention (target lesion revascularization, 83.1% versus 67.6%, P<0.01) for the overall DES group were superior to standard care in nonrandomized comparisons. Similarly, clinical benefit (81.8% versus 63.8%, P=0.02), patency (72.4% versus 53.0%, P=0.03), and freedom from target lesion revascularization (84.9% versus 71.6%, P=0.06) with provisional DES were improved over provisional BMS. These results represent >40% relative risk reduction for restenosis and target lesion revascularization through 5 years for the overall DES in comparison with standard care and for provisional DES in comparison with provisional BMS.
Conclusions: The 5-year results from this large study provide long-term information previously unavailable regarding endovascular treatment of femoropopliteal artery disease. The Zilver PTX DES provided sustained safety and clinical durability in comparison with standard endovascular treatments.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00120406.
Keywords: angioplasty; drug-eluting stents; paclitaxel; peripheral artery disease; stents.
© 2016 The Authors.
Figures



Comment in
-
Drug-Eluting Stents in the Superficial Femoral Artery: The Long and Winding Road.Circulation. 2016 Apr 12;133(15):1435-7. doi: 10.1161/CIRCULATIONAHA.116.022039. Epub 2016 Mar 11. Circulation. 2016. PMID: 26969757 No abstract available.
References
-
- Rocha-Singh KJ, Jaff MR, Crabtree TR, Bloch DA, Ansel G VIVA Physicians, Inc. Performance goals and endpoint assessments for clinical trials of femoropopliteal bare nitinol stents in patients with symptomatic peripheral arterial disease. Catheter Cardiovasc Interv. 2007;69:910–919. doi: 10.1002/ccd.21104. - PubMed
-
- Schillinger M, Sabeti S, Dick P, Amighi J, Mlekusch W, Schlager O, Loewe C, Cejna M, Lammer J, Minar E. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–2749. doi: 10.1161/CIRCULATIONAHA.107.688341. - PubMed
-
- Geraghty PJ, Mewissen MW, Jaff MR, Ansel GM VIBRANT Investigators. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg. 2013;58:386–95.e4. doi: 10.1016/j.jvs.2013.01.050. - PubMed
-
- Tepe G, Schnorr B, Albrecht T, Brechtel K, Claussen CD, Scheller B, Speck U, Zeller T. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 2015;8(1 pt A):102–108. doi: 10.1016/j.jcin.2014.07.023. - PubMed
-
- Marmagkiolis K, Hakeem A, Choksi N, Al-Hawwas M, Edupuganti MM, Leesar MA, Cilingiroglu M. 12-month primary patency rates of contemporary endovascular device therapy for femoro-popliteal occlusive disease in 6,024 patients: beyond balloon angioplasty. Catheter Cardiovasc Interv. 2014;84:555–564. doi: 10.1002/ccd.25510. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

