Impact of the U.S. Food and Drug Administration's Safety-Related Announcements on the Use of Bisphosphonates After Hip Fracture
- PMID: 26969902
- PMCID: PMC5040596
- DOI: 10.1002/jbmr.2832
Impact of the U.S. Food and Drug Administration's Safety-Related Announcements on the Use of Bisphosphonates After Hip Fracture
Abstract
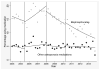
The U.S. Food and Drug Administration (FDA) issued several announcements related to potential risk of bisphosphonates including osteonecrosis of the jaw (2005), atrial fibrillation (2007), and atypical femur fracture (2010). We aimed to evaluate the impact of three FDA drug safety announcements on the use of bisphosphonates in patients with hip fracture using claims data from a U.S. commercial health plan (2004-2013). We calculated the proportion of patients in each quarter who received a bisphosphonate or other osteoporosis medication in the 6 months following hospitalization for hip fracture. Segmented logistic regression models examined the time trends. Among 22,598 patients with hip fracture, use of bisphosphonate decreased from 15% in 2004 to 3% in the last quarter of 2013. Prior to the 2007 announcement, there was a 4% increase in the odds of bisphosphonate use every quarter (OR 1.04; 95% CI, 1.02 to 1.07). After the 2007 announcement, there was a 4% decrease in the odds of bisphosphonate use (OR 0.96; 95% CI, 0.93 to 0.99) every quarter. The announcement in 2007 was associated with a significant decline in the rate of change of bisphosphonate uses over time (p < 0.001), but no impact on other osteoporosis medication use (p = 0.2). After the 2010 announcement, the odds of bisphosphonate use continued to decrease by 4% (OR 0.96; 95% CI, 0.94 to 0.98) each quarter and the odds of other osteoporosis medication use remained stable over time (OR 0.99; 95% CI, 0.96 to 1.02). The FDA safety announcement related to atrial fibrillation in 2007 was significantly associated with a decrease in bisphosphonate use among patients with hip fracture. © 2016 American Society for Bone and Mineral Research.
Keywords: BISPHOSPHONATES; HIP FRACTURE; OSTEOPOROSIS.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
Potential Conflict of Interest SCK receives research grants to the Brigham and Women’s Hospital from Pfizer, AstraZeneca, Genentech, and Lilly. DHK provides paid consultative services on geriatrics care to the Alosa Foundation, a non-profit educational organization with no relationship to any drug or device manufacturers. JMP is currently an employee of CVS Caremark. DHS has received research grants to the Brigham and Women’s Hospital from CORRONA, Astra Zeneca, Amgen, Genentech, Pfizer, and Lilly. He serves in unpaid roles on studies sponsored by Pfizer. Solomon serves on the multi-specialty board of the American Orthopaedic Association’s Own the Bone Program and on the Governing Board of the National Bone Health Alliance. Conflict of Interest: SCK receives research grants to the Brigham and Women’s Hospital from Pfizer, AstraZeneca, Genentech, and Lilly. DHK provides paid consultative services on geriatrics care to the Alosa Foundation, a non-profit educational organization with no relationship to any drug or device manufacturers. HM, WE, JMF, and JMP have nothing to disclose. DHS receives salary support through research grants to the Brigham and Women’s Hospital from CORRONA, Astra Zeneca, Amgen, Genentech, Pfizer, and Lilly. He serves in an unpaid role on a trial sponsored by Pfizer unrelated to the current study. He serves on the multi-specialty board of the American Orthopaedic Association’s Own the Bone Program and on the Governing Board of the National Bone Health Alliance.
Figures
References
-
- National Osteoporosis Foundation. Cinician’s guide to prevention and treatment of osteoporosis Secondary Cinician’s guide to prevention and treatment of osteoporosis. 2013 http://nof.org/files/nof/public/content/resource/913/files/580.pdf.
-
- Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis Medication Use after Hip Fracture in U.S. Patients between 2002 and 2011. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2014 doi: 10.1002/jbmr.2202. published Online First: Epub Date. - DOI - PMC - PubMed
-
- Safety Alerts for Human Medical Products - Aredia (pamidronate disodium), Zometa (zoledronic acid) Secondary Safety Alerts for Human Medical Products - Aredia (pamidronate disodium), Zometa (zoledronic acid) 2005 http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHuma....
-
- Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa) Information. Secondary Bisphosphonates (marketed as Actonel, Actonel+Ca, Aredia, Boniva, Didronel, Fosamax, Fosamax+D, Reclast, Skelid, and Zometa) Information. 2015 Jul 8; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPa....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

