Diffusible iodine-based contrast-enhanced computed tomography (diceCT): an emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues
- PMID: 26970556
- PMCID: PMC5341577
- DOI: 10.1111/joa.12449
Diffusible iodine-based contrast-enhanced computed tomography (diceCT): an emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues
Abstract
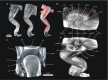
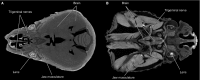
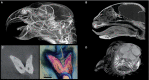
Morphologists have historically had to rely on destructive procedures to visualize the three-dimensional (3-D) anatomy of animals. More recently, however, non-destructive techniques have come to the forefront. These include X-ray computed tomography (CT), which has been used most commonly to examine the mineralized, hard-tissue anatomy of living and fossil metazoans. One relatively new and potentially transformative aspect of current CT-based research is the use of chemical agents to render visible, and differentiate between, soft-tissue structures in X-ray images. Specifically, iodine has emerged as one of the most widely used of these contrast agents among animal morphologists due to its ease of handling, cost effectiveness, and differential affinities for major types of soft tissues. The rapid adoption of iodine-based contrast agents has resulted in a proliferation of distinct specimen preparations and scanning parameter choices, as well as an increasing variety of imaging hardware and software preferences. Here we provide a critical review of the recent contributions to iodine-based, contrast-enhanced CT research to enable researchers just beginning to employ contrast enhancement to make sense of this complex new landscape of methodologies. We provide a detailed summary of recent case studies, assess factors that govern success at each step of the specimen storage, preparation, and imaging processes, and make recommendations for standardizing both techniques and reporting practices. Finally, we discuss potential cutting-edge applications of diffusible iodine-based contrast-enhanced computed tomography (diceCT) and the issues that must still be overcome to facilitate the broader adoption of diceCT going forward.
Keywords: Lugol's iodine; X-ray micro-CT scanning; alcoholic iodine; destaining; radiographic contrast agents; three-dimensional imaging.
© 2016 The Authors. Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Figures





References
-
- Balanoff AM, Bever GS, Colbert MW et al. (2015) Best practices for digitally constructing endocranial casts: examples from birds and their dinosaurian relatives. J Anat doi: 10.1111/joa.12378. - DOI - PMC - PubMed
-
- Buytaert J, De Greef D, Aerts P, et al. (2014) Volume shrinkage of bone, brain and muscle tissue in sample preparation for micro‐CT and light sheet florescence microscopy (LSFM). Microsc Microanal 20, 1208–1217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

