Sex-, stress-, and sympathetic post-ganglionic-dependent changes in identity and proportions of immune cells in the dura
- PMID: 26970607
- PMCID: PMC5023503
- DOI: 10.1177/0333102416637832
Sex-, stress-, and sympathetic post-ganglionic-dependent changes in identity and proportions of immune cells in the dura
Abstract
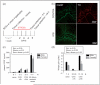
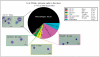
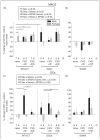
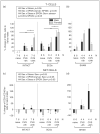
Aim of investigation Due to compelling evidence in support of links between sex, stress, sympathetic post-ganglionic innervation, dural immune cells, and migraine, our aim was to characterize the impacts of these factors on the type and proportion of immune cells in the dura. Methods Dural immune cells were obtained from naïve or stressed adult male and female Sprague Dawley rats for flow cytometry. Rats with surgical denervation of sympathetic post-ganglionic neurons of the dura were also studied. Results Immune cells comprise ∼17% of all cells in the dura. These included: macrophages/granulocytes ("Macs"; 63.2% of immune cells), dendritic cells (0.88%), T-cells (4.51%), natural killer T-cells (0.51%), natural killer cells (3.08%), and B-cells (20.0%). There were significantly more Macs and fewer B- and natural killer T-cells in the dura of females compared with males. Macs and dendritic cells were significantly increased by stress in males, but not females. In contrast, T-cells were significantly increased in females with a 24-hour delay following stress. Lastly, Macs, dendritic cells, and T-cells were significantly higher in sympathectomized-naïve males, but not females. Conclusions It may not only be possible, but necessary to use different strategies for the most effective treatment of migraine in men and women.
Keywords: Headache; autonomic; inflammation; meninges.
Figures




References
-
- Levy D. Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading depression. Curr Pain Headache Rep. 2012;16:270–277. - PubMed
-
- Olesen J, Burstein R, Ashina M, et al. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8:679–690. - PubMed
-
- Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64(10 Suppl. 2):S9–S15. - PubMed
-
- Reuter U, Bolay H, Jansen-Olesen I, et al. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001;124:2490–2502. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

